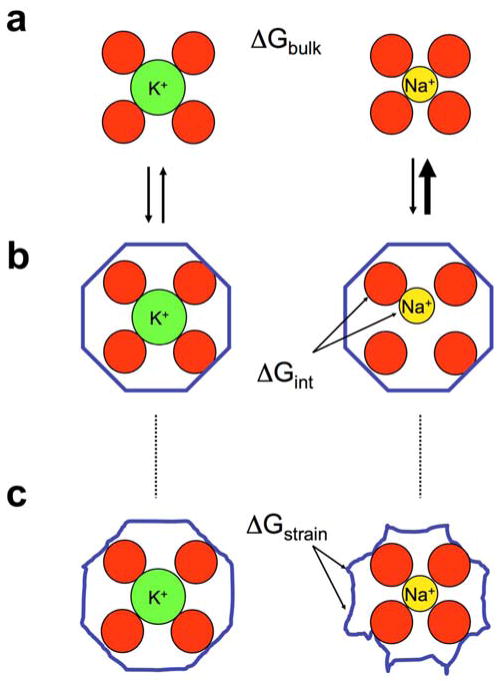
Figure 2.
Schematic illustration of fundamental concepts in thermodynamic ion binding selectivity. In (a), the K+ and Na+ ions are pictured in bulk solution with their first hydration shell. The difference in hydration free energy ΔGbulk between these two cations is ~18 kcal/mol. Binding to a rigid host (b) with a cavity size matching precisely a K+ ion (left) does not provide a favorable environment for the smaller Na+ (right). In this case, selectivity arises from the poor coordination interaction free energy ΔGint between the ion and its rigid host. This is the classical snug-fit mechanism of host-guest chemistry. However, selectivity may also be achieved by a flexible host (c) that is able to deform and adapt to both K+ and Na+ ion if there is a sufficient build-up of strain energy ΔGstrain (illustrated as a deformation of the blue molecular scaffolding). Reproduced with permission from (Noskov and Roux, 2007).