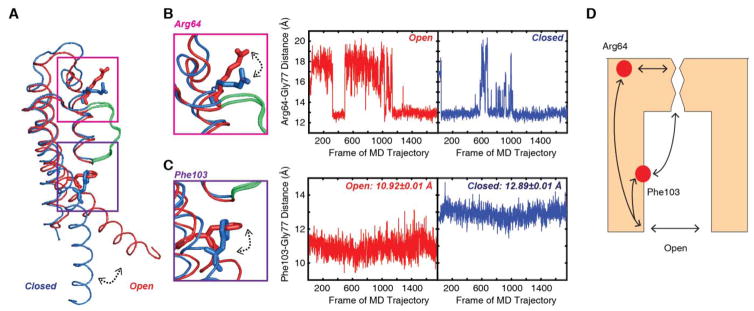
Fig. 5. Coupling of the intracellular gate involves residues Arg64 and Phe103 near the selectivity filter.
(A) Overlay of the open (red) and closed (blue) monomer of KcsA. The selectivity filter is shown in green. (B) When the intracellular gate is closed, the Arg64 residue is closer to the selectivity filter, as shown by the blue trace of the Arg64-Gly77 distance which measures the distance between the Arg Cζ and the Gly77 carbonyl oxygen. When the gate opens, the distance between the Arg64 and the Gly77 residues increases. (C) Likewise, the distance between the Phe103 Cβ and the Gly77 carbonyl oxygen varies between closed and open states. In the closed state, the Phe103 and Gly77 is further apart, with a distance of 12.89 ± 0.01 Å. When the transmembrane helix rotates as the intracellular gate opens, the distance between these two residues decreases to values of 10.92 ± 0.01 Å. (D) Illustration of proposed mechanism for conformational changes in the selectivity filter upon gate opening. The communication pathway between the gate and the selectivity filter is facilitated by residues along the gating and pore helices, including Phe103 and Arg64.