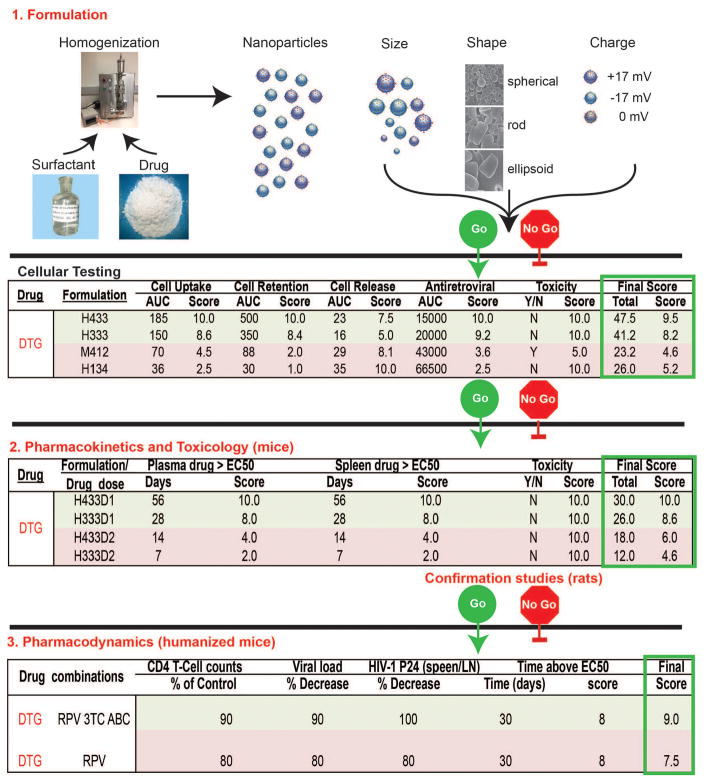
Figure 1.
Go no-go criteria for the development of dolutegravir (DTG) LASER ART. While DTG is provided as an example it represents one of many ARVs being developed into long acting formulations. Using high-pressure homogenization (H) or wet milling (M) ARVs are packaged into particles with surfactant and ligand coatings designed to target circulating mononuclear phagocytes (MP; dendritic cells, monocytes and macrophages). The size, shape, charge and surfactant coating of the particles facilitate optimal MP uptake into subcellular autophagosomes (numbers under formulation are the poloxamer, particle shape and size). MPs serve as drug depots and scavenge particles serving as delivery vehicles. Laboratory cell-based tests are used to measure MP particle uptake, retention, and release of nanoART and determine antiretroviral activity (defined by area under the curve, AUC, for each activity over time) and cytotoxicity. A ranked scoring system for the made formulations determines those best suited to be moved forward for PK testing in mice and rats at two doses (D1 and 2). Select formulations are used for pharmacodynamic tests to demonstrate extended antiretroviral efficacy in humanized rodent models of HIV disease. Final therapeutic scores and consideration for human use are dependent upon toxicity measures, dosing, and end organ toxicities that include no adverse hematologic, renal, hepatic, immune or other systemic events. Pharmacodynamics screens are used to assess clearance of virus from its tissue reservoirs by ultrasensitive viral RNA and DNA detection systems. A range of drug tissue distribution, viral, pharmacologic and immune tests with end organ histology evaluations are performed to determine drug efficacy.