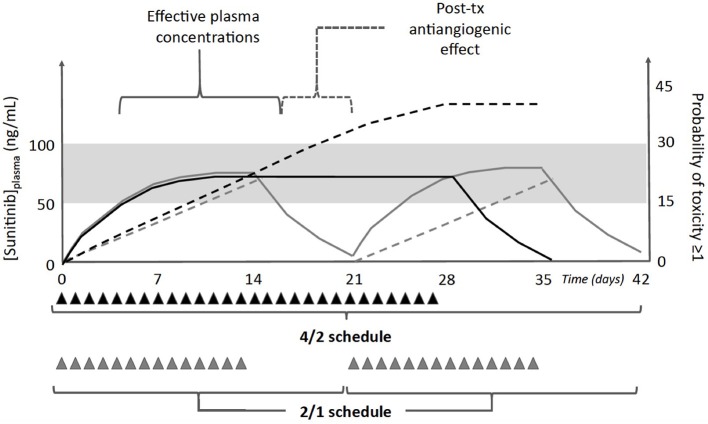
Figure 1.
Schematic representation of plasma concentrations (solid lines) and probability of toxicities grade ≥1 (dashed lines) associated with administration of sunitinib 50 mg/day (triangles) according to the standard 4/2 (black lines/triangles), or the alternative 2/1 schedule (gray lines/triangles). Both regimens have the same dose intensity, but the increased severity of toxicities is associated with the standard schedule. Furthermore, there is the evidence that sunitinib effects may extend up to 1 week after the last daily dose (post-tx antiangiogenic effects), hence the 1 week of rest in the 2/1 schedule could be beneficial for patients while ensuring the recovery from mild toxicities. The gray area represents the therapeutic range of sunitinib plasma concentrations (50–100 ng/mL).