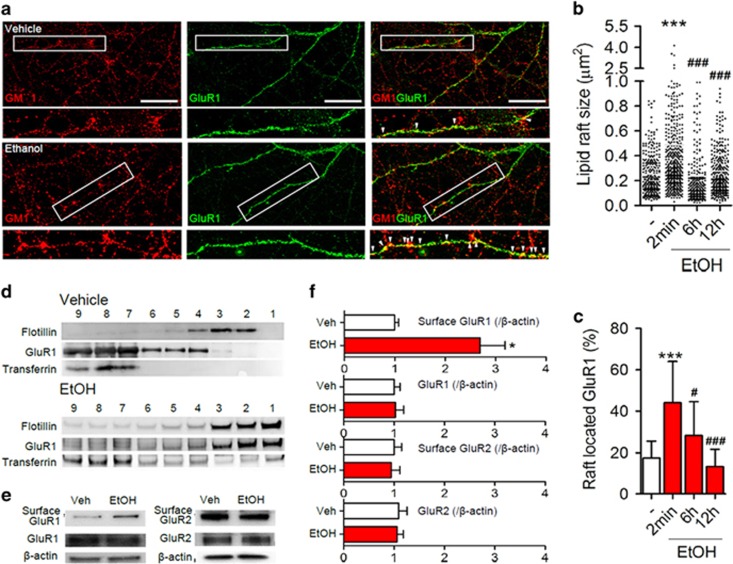
Figure 3.
EtOH modifies the biophysical properties of cellular membranes and redistributes GluR1 to microdomains. (a) Representative immunofluorescent images showing EtOH facilitated a rapid (2 min) redistribution of the microdomains (GM1+, red) into enlarged clusters along dendrites, simultaneously resulted in redistributions of GluR1 (green) with clusters located to GM1+ microdomains (arrowheads indicate GM1/GluR1-co-localized microdomains). (b) Size of individual GM1+ microdomains, and (c) number of GluR1 located to GM1+ microdomains (n=276–440 microdomains from a minimum of 21 dendrites and 3 separate cultures per condition). (d) Representative immunoblots showing detergent-resistant membrane microdomains isolated by density centrifugation containing a membrane microdomain-enriched protein Flotillin (largely located to the more buoyant lipid-rich fractions, 1–3), a non-microdomain protein Transferrin (largely located outside of membrane microdomains, fractions 7–9) and GluR1. EtOH (2 min) induced a redistribution of GluR1 to membrane microdomains. (e) Representative immunoblots of surface biotin protein labeling and immunoprecipitation with subsequent immunoblotting for GluR1 or GluR2. (f) Quantitative analysis of immunoblots showing that EtOH specifically increased surface GluR1 but not total GluR1. Data are presented as mean±s.d. EtOH, ethanol. *P<0.05, ***P<0.001 compared with vehicle; #P<0.05, ###P<0.001 compared with EtOH. Scale bar, 20 μm.