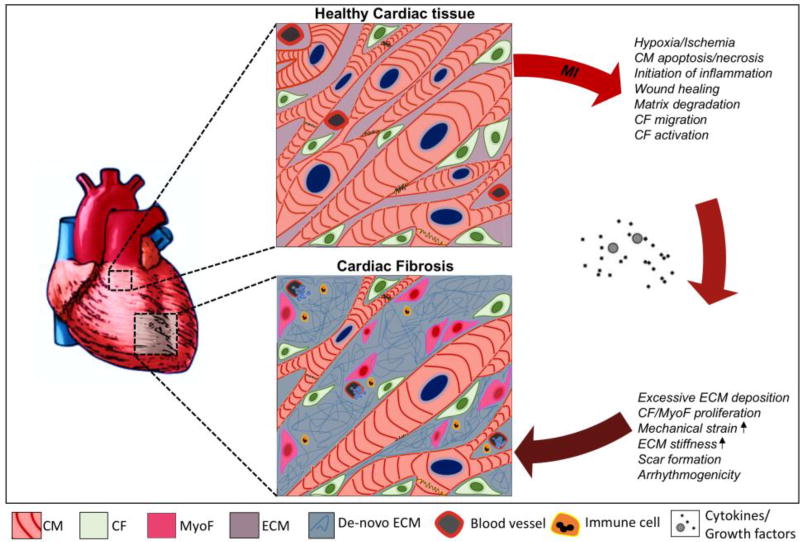
Figure 1. Schematic illustration of the pathophysiological changes during fibrotic cardiac remodeling.
Healthy myocardial tissue consists of a network of cardiomyocytes (CM) and quiescent cardiac fibroblasts (CF) that are interspersed within the extracellular matrix (ECM). After myocardial injury (eg. myocardial infarct (MI)), CMs die and a reparative inflammatory and wound healing process is initiated by the release of various cytokines and growth factors (such as transforming growth factor-β1, angiotensin-II etc.). This results in the activation of cardiac fibroblasts into cardiac myofibroblasts (MyoF). These cells and other resident cardiac fibroblasts are responsible for an excessive and prolonged synthesis and deposition of de-novo ECM proteins (eg. collagen-I, fibronectin, laminin). This results in scarring of the heart tissue and leads to the deterioration of ventricular function followed by diastolic and systolic ventricular dysfunction and may eventually lead to life threatening arrhythmogenicity or heart failure.