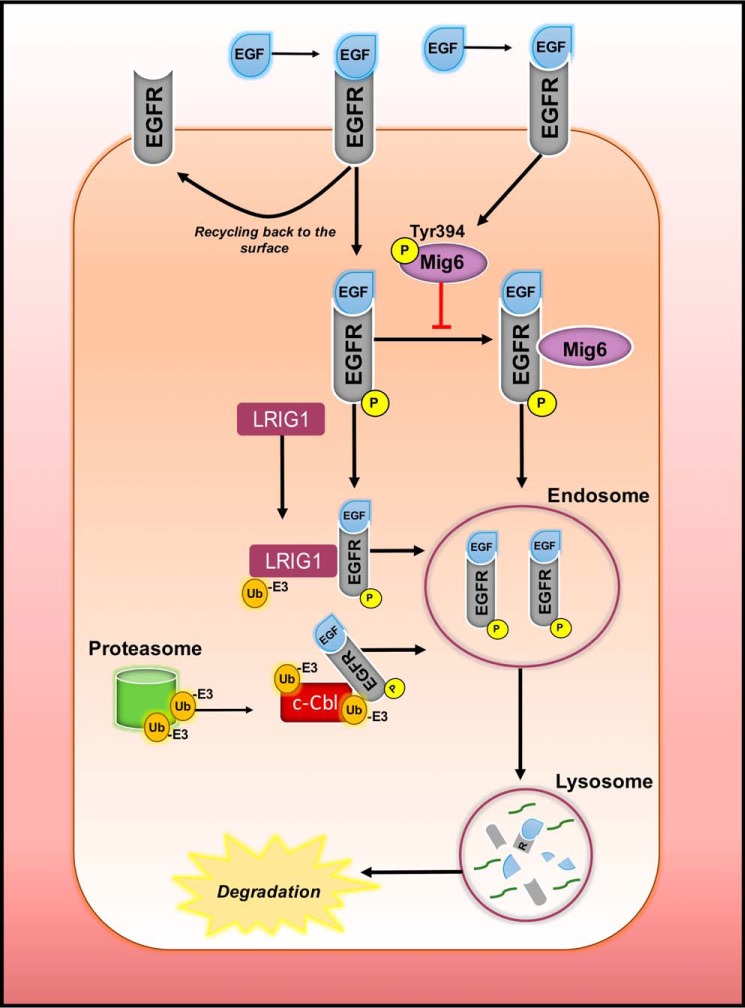
Figure 3.
Prospective mechanisms of degradation of EGFR. Once bound to the ligand EGF, EGFR is rapidly activated and subsequently internalized into endosomes, from which it can either be recycled back to the surface or proceed to lysosomal degradation. A mechanism that explains the degradation process is via E3 ubiquitin ligase (Ub−E3) that has been shown to facilitate the endocytosis of EGFR. The protein leucine-rich repeat and immunoglobulin-like domain 1 (LRIG1) may also be involved by simultaneously ubiquitinating itself and EGFR and recruiting c-Cbl to sort them into endosomes. There has also been evidence of participation of the proteasome through the release of Ub−E3 to further facilitate the degradation process. Additionally, a known inhibitor of EGFR is the mitogen-inducible gene 6 (MIG6) which binds to the carboxyl-terminal lobe of EGFR to deter its signaling. However, in tumor cells, this is unable to occur, as phosphorylation of MIG6 by EGFR at Tyr-394 stops the binding of MIG6 to EGFR, inhibiting receptor degradation.