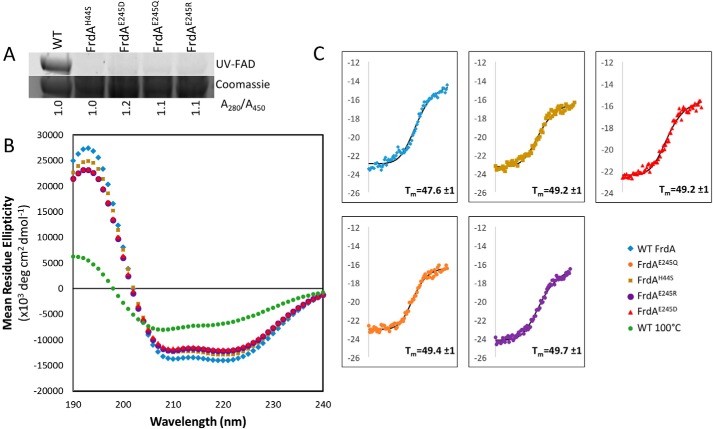
Figure 6.
Circular dichroism spectroscopy of wild-type and variant FrdA subunits. A, measurement of flavin in wild-type and variant FrdA subunits (∼67 kDa). The quantity of flavin covalently associated with FrdA was measured by separating lysates on SDS-PAGE and measuring FAD fluorescence following illumination with UV light. The relative quantity of FAD associated with wild-type and variant FrdA subunits was assessed by measuring the A280/A450 ratio of purified proteins. This value was normalized to 1.0 for wild-type protein. The ratio of A280/A450 was similar in wild-type and variant subunits, indicating that these had a similar amount of flavin associated with each subunit. As in gel UV detection indicates that flavin is not covalently associated with the subunits, the tightly associated FAD must be non-covalently bound, likely via specific binding to the active site. B, far-UV CD spectrum of wild-type FrdA compared with spectra for the FrdAH44S variant, the FrdAE245 variants, and FrdA incubated at 100 °C for 2 h. C, representative traces of thermal CD of wild-type FrdA, FrdAH44S, FrdAE245D, FrdAE245Q, and FrdAE245R. The average Tm values are indicated.