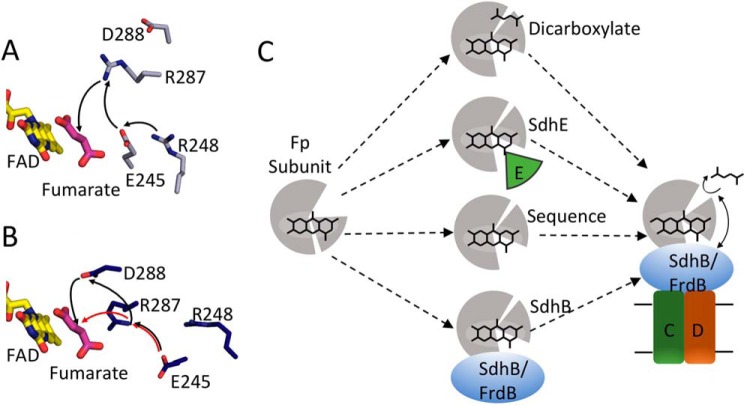
Figure 9.
Model for side chains in covalent flavinylation. A, active site organization in wild-type QFR with fumarate bound (gray, PDB entry 3P4P (38)). FAD (yellow) and fumarate (pink), and side chains or FrdA245, FrdA248, FrdA287, and FrdA288 are shown as sticks. The black arrows indicate the direction of proton transfer during fumarate reduction. B, the same view for the FrdA-SdhE-R8BpF model (blue). The black and red arrows indicate two potential alternate proton transfer pathways after reorganization of the active site as a result of domain rotation in the model. C, scheme for organization of the flavoprotein (gray) active site to promote autocatalysis of the covalent flavin bond. In this model, closure of the capping domain after binding of flavin (represented as stick model of the isoalloxazine ring) and proper orientation of the attachment site can be facilitated in several ways: (i) binding of dicarboxylate; (ii) binding of the assembly factor; (iii) enhanced stability and preorganization by sequence in thermophilic bacteria; (iv) binding of the iron-sulfur subunit. After this organization, covalent flavinylation can occur (indicated by additional bond) followed by complex assembly.