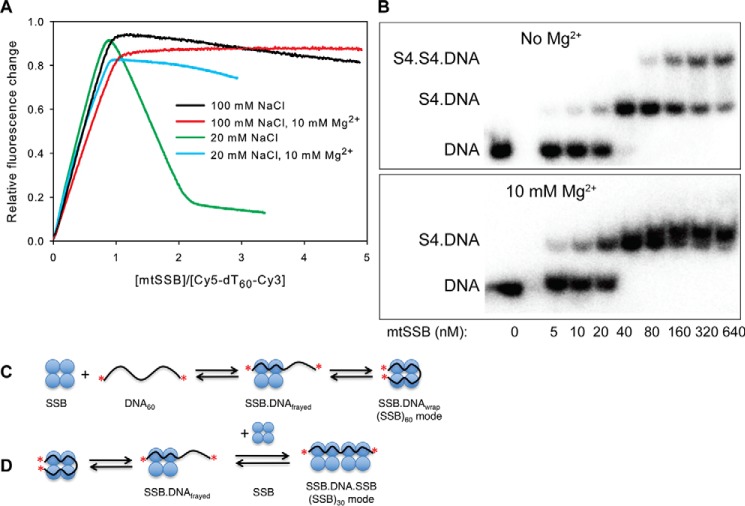
Figure 2.
mtSSB binds to ssDNA in two different binding modes, modulated by concentrations of NaCl and Mg2+. A, a fixed concentration of Cy5-dT60-Cy3 (40 nm) was titrated with increasing concentrations of mtSSB tetramer while monitoring the FRET fluorescence (emission from Cy5 when exciting Cy3) in buffer A with 100 mm NaCl (black), with 100 mm NaCl and 10 mm Mg2+ (red), with 20 mm NaCl (green), or with 20 mm NaCl and 10 mm Mg2+ (blue). The data (except for the red line) showed a biphasic nature, suggesting the formation of two types of complexes, (SSB)60 and (SSB)30, which were characterized by high and low FRET values, respectively. B, effect of Mg2+ on the binding modes of mtSSB protein to dT60 measured by gel shift. The reaction solutions contained 30 nm 32P-labeled dT60 and the indicated concentrations of mtSSB (0–640 nm) in buffer A with 100 mm NaCl (−Mg2+) or with 100 mm NaCl and 10 mm MgCl2 (+Mg2+). The products were separated by polyacrylamide gel electrophoresis with the running buffer containing the same concentration of Mg2+ as in the reaction solutions and were then visualized by autoradiography. C, schematic showing the binding and wrapping of DNA around mtSSB. The red asterisks illustrate the Cy5 and Cy3 labels. D, schematic showing binding of a second mtSSB to the SSB–DNA complex to transition from the (SSB)60 mode to the (SSB)30 mode. For reasons described under “Results,” we illustrate this reaction involving the binding of SSB to a partially frayed intermediate in equilibrium with the fully wrapped complex.