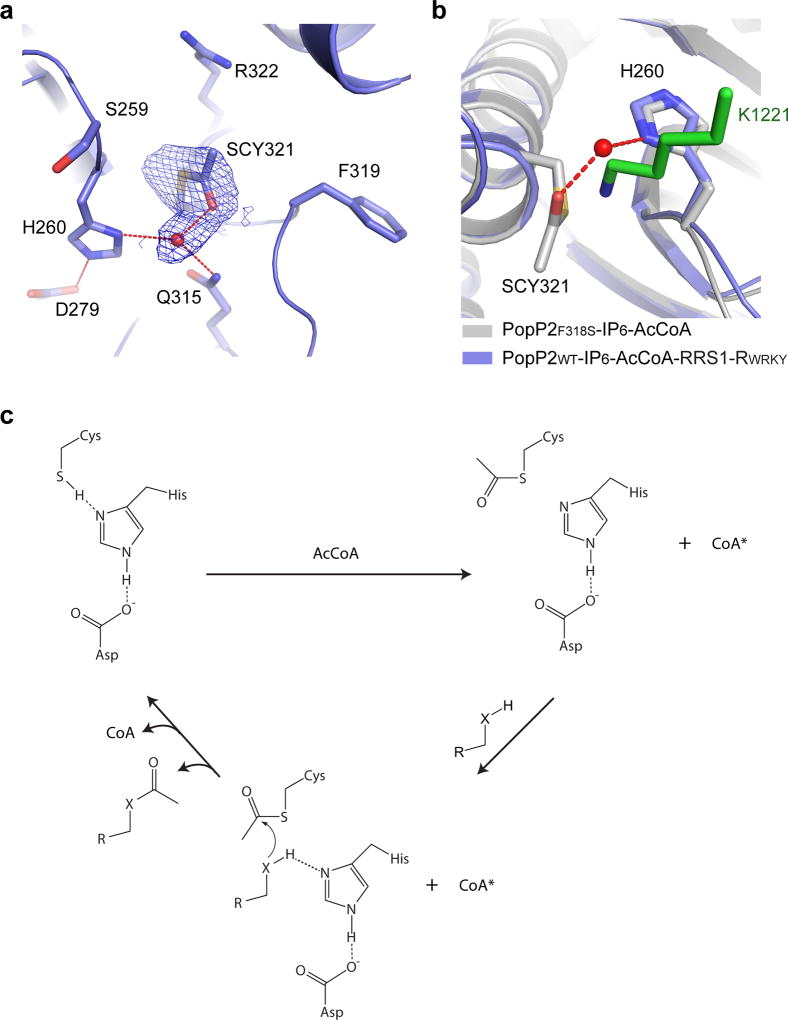
Figure 6. A model for enzymatic catalysis of YopJ effectors.
a, Close-up view of the catalytic site in the crystal structure of PopP2F318S – IP6 – AcCoA, with the 2Fo-Fc omit map of the acetyl-cysteine (SCY321) contoured at 1.0σ level. The water molecule is shown as a red sphere and the hydrogen bonds are shown as dashed lines. b, The active site of PopP2F318S – IP6 – AcCoA overlaid with that of PopP2WT– IP6 – AcCoA– RRS1-RWRKY. The target lysine is shown in green stick. c, A model for the catalytic steps of YopJ effector-mediated acetylation. *The adenosine moiety of CoA remains bound to PopP2, whereas the pantetheine arm is released from the catalytic center. “X” denotes an NH2 group or oxygen atom.