Fig. 5.
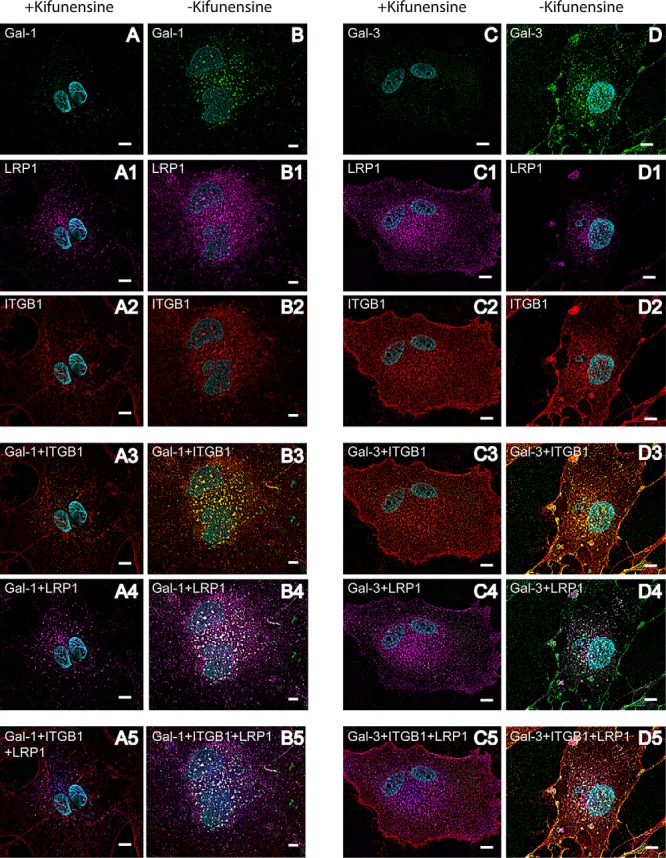
Complex-type N-glycosylation of galectin-interactors is necessary for galectin-induced cross-linking of LRP1 and ITGB1 on the cell surface of mesenchymal RPE cells. Immunocytochemical staining of human RPE cells, pretreated with 10 μm kifunensine. Before fixation cells were pretreated with biotinylated Gal-1 or Gal-3 for 30 min. Galectin binding was visualized with streptavidin-AlexaFluor488 (green) (A–D), LRP1 by AlexaFluor647 (magenta) (A1–D1), and ITGB1 by AlexaFluor568 (red) (A2–D2). Overlay of LRP1 and galectin staining patterns is visible in white (A4–D4) on the overlay of ITGB1 and galectin staining patterns are in yellow (A3–D3). For visualization of the clustering of galectin, LRP1 and ITGB1, LRP1 staining was changed in silico to blue, and the overlay is seen in white (A5–D5). Whereas addition of exogenous galectin led to clear co-localization of LRP1 and ITGB1 on human RPE cells not treated with kifunensine (B–B5 and D–D5), no cross-linking could be observed in RPE cells treated with kifunensine (A–A5 and C–C5). Representative images from two independent experiments are shown. Scale bar, 10 μm.
