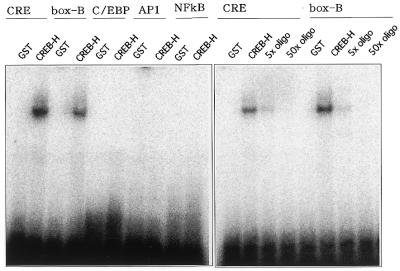
Figure 5.
DNA-binding ability of CREB-H analyzed by gel-shift assays. A fusion protein of GST and a CREB-H mutant lacking the putative transmembrane domain was produced in bacteria and purified by glutathione–Sepharose 4B. Oligonucleotides representing CRE, C/EBP, AP1, NF-κB and box-B elements were annealed and labeled with [γ-32P]ATP and incubated with the GST–CREB-H fusion protein. Purified non-fused GST was used in a control lane. Competitive assays for CRE and the box-B element were performed with 5- and 50-fold excesses of unlabeled oligonucleotides for CRE and the box-B element, respectively.