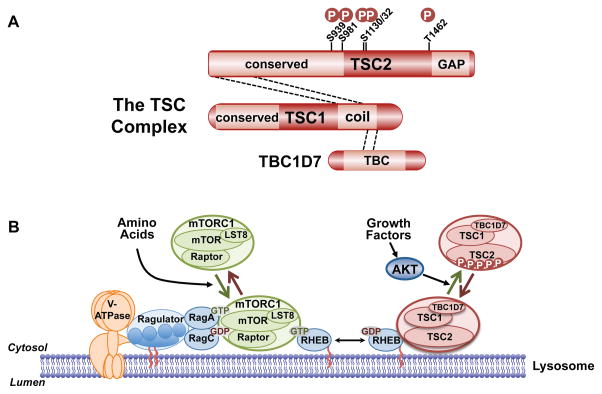
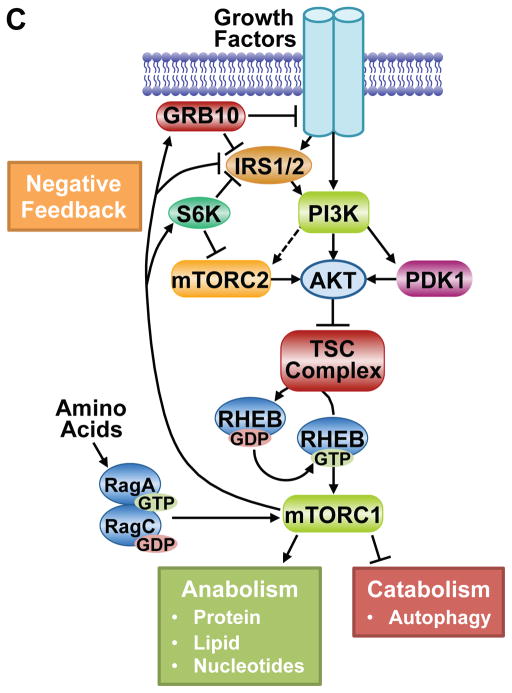
Figure 5. Regulation of mTORC1 via the TSC complex and downstream functions of mTORC1.
A. Schematic of the TSC complex components, their regions of association (dashed lines), and Akt phosphorylation sites on TSC2. Conserved domains of unknown function and GAP, coiled-coil, and TBC domains of the components are shown. B. Model of signal integration by growth factors and amino acids for regulation of mTORC1. The Rag heterodimer interacts with the Ragulator and V-ATPase at the lysosomal surface, and amino acids promote mTORC1 binding to this complex. The TSC complex maintains Rheb in the GDP-bound state. Growth factor-stimulated Akt phosphorylates TSC2, resulting in dissociation from the lysosomal surface, allowing Rheb to become GTP loaded and activate mTORC1. C. The PI3K-mTOR signaling pathway, depicting downstream functions and feedback regulation.