Figure 1. Experimental strategy.
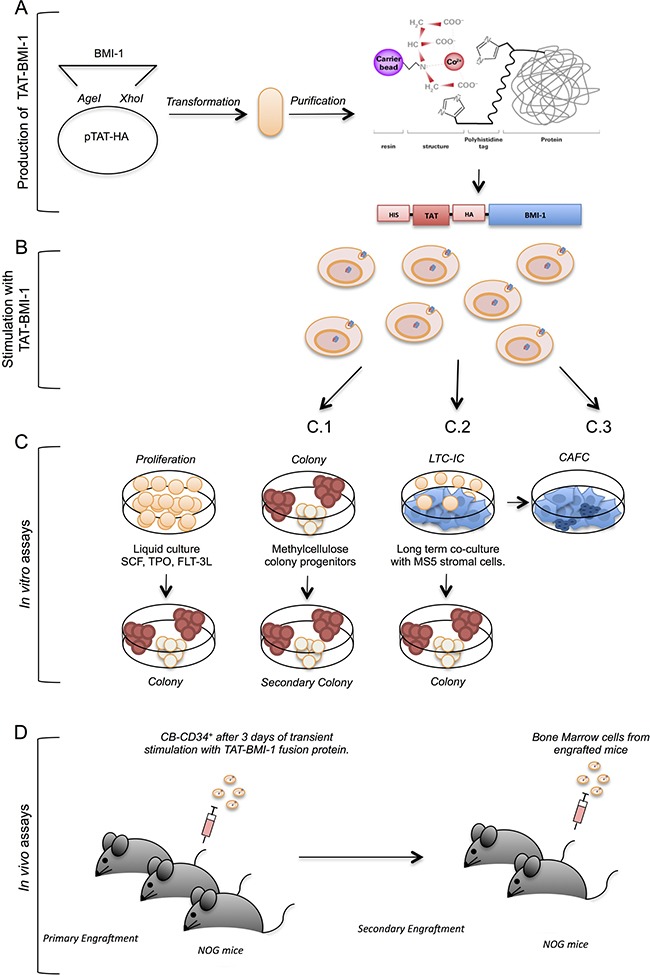
This figure depicts the experimental workflow: (A) TAT-fusion proteins were produced in bacteria using the pTAT-HA plasmid, and purified by affinity chromatography; (B) the purified TAT-fusion protein were used to stimulate primary CB-CD34+ cells in the presence of early-acting hemopoietins (SCF, FL, TPO); (C) the cells thus stimulated were maintained in cytokine-driven cultures to monitor the total cell expansion and the numbers of colony-forming cells in these cultures (C.1), as well as the presence of self-renewing progenitors through secondary colony assays (C.2), or assayed in stromal co-cultures (C.3) to assess numbers of early myeloid progenitors (CAFCs); (D) CB-CD34+ cells treated with TAT-fusion proteins were assayed in vivo by xenotransplant of severely immunocompromised (NOG) mice following sublethal myeloablation with busulfan, and the engraftment of human cells was monitored in the blood and bone marrow of these animals by flow-cytometry; secondary transplants were performed to measure the presence of self-renewing long-term hematopoietic stem cells.
