Figure 4. Wild-type p53 represses TGFβ/SMAD3-induced NOX4 promoter activity.
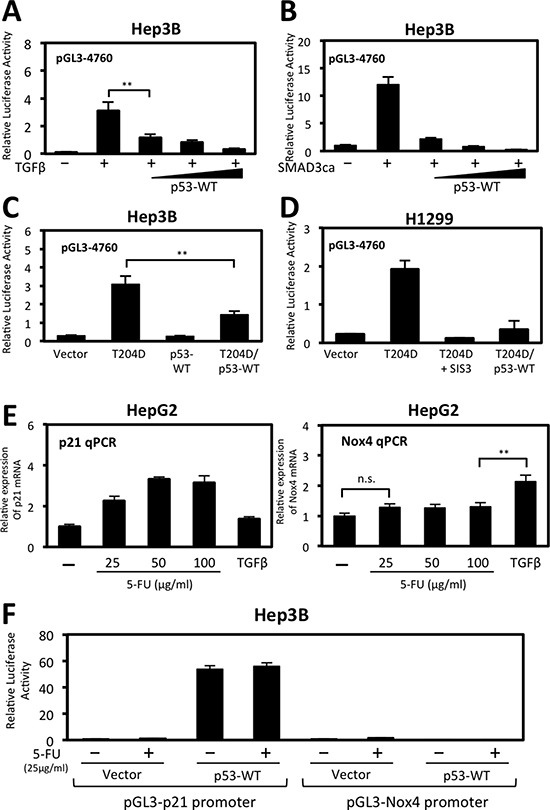
(A) Hep3B cells were co-transfected with NOX4 promoter-luciferase reporter plasmid pGL3-NOX4 (-4760) (0.5 μg) and vector control (0.5 μg) or increasing amounts of p53-WT (0.1, 0.3, or 0.5 μg) for 24 hours then either treated with TGFβ (5 ng/ml) for 24 hours or left untreated. Total cell lysates were collected 48 hours post-transfection and assayed for luciferase activity by luminescence (n = 4, in triplicate). (B) Hep3B cells were co-transfected with NOX4 promoter reporter and vector control or constitutively active SMAD3 (0.5 μg). Wild-type p53 was co-transfected at increasing concentrations as in panel A. Forty-eight hours after transfection, total cell lysates were assayed as in panel A (n = 3, in triplicate). (C) Hep3B cells were co-transfected with pGL3-NOX4 (-4760) and either vector control, constitutively active TGFβR1 (T204D), p53-WT, or T204D and p53-WT. After 48 hours, total cell lysates were collected for luciferase activity (n = 3, in triplicate). (D) H1299 cells transfected with the pGL3-NOX4 (-4760) promoter-reporter were co-transfected with either vector control, T204D, or with T204D and p53-WT. Twenty-four hours after transfection, SIS3 (10 μM) was added for an additional 24 hours. Cell lysates were collected and assayed for luciferase activity. (E) HepG2 cells were treated with increasing concentrations of 5-fluorouracile (5-FU) (25, 50, or 100 μg/ml) or TGFβ (5 ng/ml) for 24 hours. Real-time quantitative PCR (qPCR) analysis of p21/CDKN1A mRNA expression (right panel) or NOX4 mRNA (left panel) was determined using gene-specific primers for human p21 or NOX4. Results are described as p21 or NOX4 mRNA expression relative to untreated control and normalized to GAPDH (n = 3, in triplicate). (F) Hep3B cells were co-transfected with p21 promoter reporter or pGL3-NOX4 (-4760) plasmids and control vector or p53-WT plasmids for 24 hours followed by 5-FU (25 μg/ml) treatment for an additional 24 hours. Total cell lysates were collected and assayed for luciferase activity (n = 3, in triplicate). Significance values are indicated as **P-value < 0.01 or n.s. (not significant).
