Figure 5.
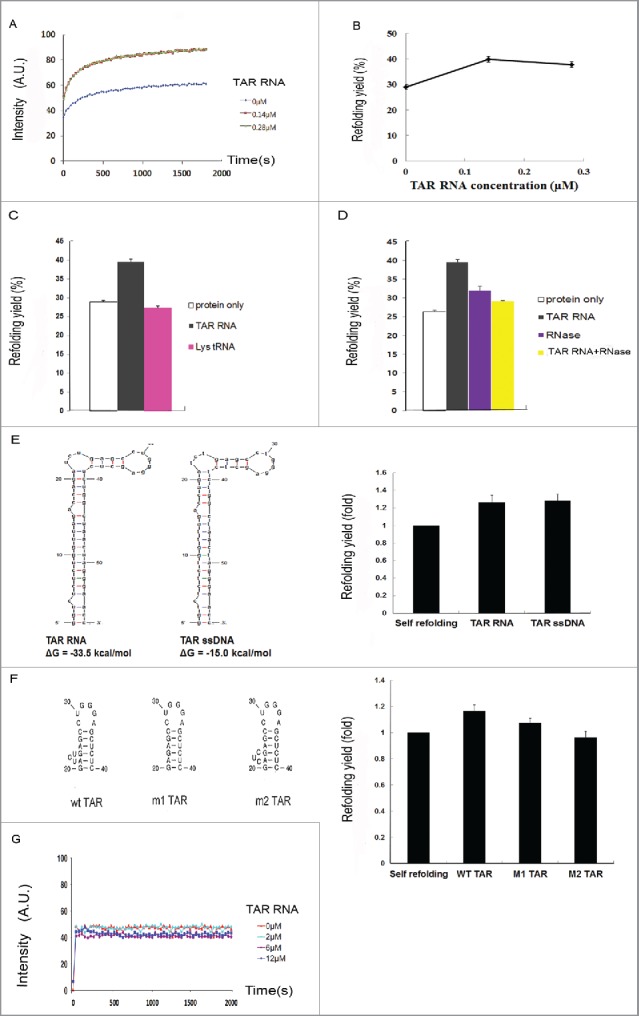
In vitro refolding of Tat-EGFP. (A) Time course of fluorescence intensity and (B) refolding yield as measured by EGFP fluorescence at different TAR RNA concentrations. Error bars represent the standard error of the means. The denatured Tat-EGFP in 6M guanidine-HCl was diluted to a final concentration of 0.14 μM in the refolding buffer containing different concentrations of TAR RNA. Refolding of Tat-EGFP (C) in the presence of cognate TAR RNA or irrelevant Lys tRNA, or (D) after RNase treatment (25 μg/mL RNase A at 37°C for 30 min). The concentration ratio of Tat-EGFP to RNA was adjusted to 1:1. Error bars represent the standard error of the means. To verify it TAR RNA binding is an important factor for HIV-1 Tat refolding, a mutation of the TAR RNA binding site was constructed. (E) Secondary structures and free energies of TAR RNA and single-stranded (ssDNA) were predicted by the Mfold program68. The effects on Tat-EGFP refolding are compared. The concentration of Tat protein, ssDNA and TAR RNA was 0.14 μM. The folding yield is shown as relative to the self-refolding of Tat-EGFP only, which was taken as 1. (F) The refolding of Tat-EGFP in the presence of TAR mutants. (G) Time course of refolding of EGFP as control at various concentrations of TAR RNA.
