Introduction
Early and effective treatment of schizophrenia may slow disease progression and improve overall patient outcomes.1 In addition, interventions specifically targeting cognitive deficits may prevent chronic disability2
In individuals with first-episode schizophrenia, greater improvements have been reported with long-acting injections vs oral antipsychotics (APs)3,4
The Disease Recovery Evaluation and Modification (DREaM) study is examining whether paliperidone palmitate once-monthly (PP1M) followed by paliperidone palmitate once-every-3-months (PP3M) injections can slow disease progression and possibly modify the course of schizophrenia compared with oral APs in subjects with recent-onset psychosis (schizophrenia or schizophreniform disorder) by tracking changes in cognition, functioning, and intracortical myelin volume and by tracking treatment failures
Key innovations of the DREaM study include double randomization of matched-control subjects in a delayed-start design. This study design is used to distinguish between a treatment’s effect on symptom improvement and potential disease modification5
We describe the baseline demographics and clinical characteristics of early enrollees to the DREaM study
These characteristics are compared to those of subjects enrolled in a trial evaluating a similar population: the Recovery After an Initial Schizophrenia Episode (RAISE) study, a multisite, randomized controlled trial in subjects with first-episode psychosis6
Methods
Study Designs
DREaM (NCT02431702)
DREaM is a prospective, matched-control, double-randomized, open-label, flexible-dose study in subjects with recent-onset schizophrenia or schizophreniform disorder that compares disease progression and disease interception following treatment with PP1M/PP3M or oral APs
Subjects aged 18 to 35 years with a DSM-5 diagnosis of schizophrenia or schizophreniform disorder and first psychotic episode within 2 years of enrollment are eligible
Participating sites include academic- and community-based clinics both with and without established clinics that specialize in first-episode psychosis
DREaM includes three treatment phases (Figure 1)
Figure 1.
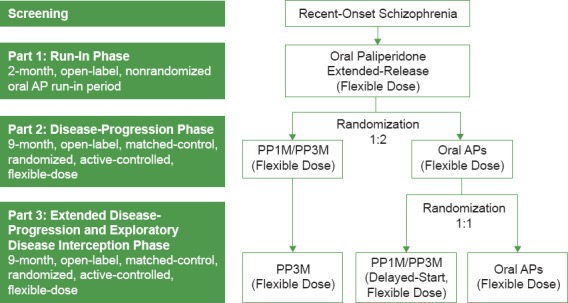
DREaM Study Design
RAISE
Subjects were aged 15 to 40 years with a DSM-IV diagnosis of schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, or psychotic disorder not otherwise specified
All participants had experienced only one episode of psychosis and had received ≤6 months of AP medications in their lifetime
Participating sites included 34 community mental health centers in the United States
Academic centers or sites with existing first-episode psychosis programs were excluded from participation6
DREaM Screening/Baseline Efficacy Assessments
- The following assessments were performed at either screening (visit 1) or baseline (Part 1: visit 2, day 1):
- ○ Baseline demographics and clinical characteristics
- ○ MATRICS Consensus Cognitive Battery (MCCB)7: Measured key cognitive domains relevant to schizophrenia and related disorders
- ○ Personal and Social Performance (PSP) scale8: Assessed personal and social functioning within the past month
- ○ Clinical Global Impression of Severity (CGI-S) scale9: Rated the severity of the subject’s overall clinical condition on a 7-point scale (1 = not ill, 7 = extremely ill)
- ○ Clinician-Rated Dimensions of Psychosis Symptom Severity (CRDPSS)12: An 8-item measure that assesses the severity of mental health symptoms that are important across psychotic disorders, such as delusions, hallucinations, disorganized speech, abnormal psychomotor behavior, negative symptoms, impaired cognition, depression, and mania, on a 5-point scale (0 = none, 4 = present and severe)
Statistical Analyses
Baseline demographics and clinical characteristics were generated for the DREaM study using descriptive statistics. In some cases where data were available, demographics and clinical characteristics were compared between the DREaM and RAISE studies
Study comparisons for available data points were carried out using either the chi-squared test for categorical data or the t-test for continuous data points
Results
DREaM vs RAISE Study
The DREaM study had enrolled 96 subjects as of April 4, 2017
Baseline demographics in the DREaM and RAISE studies were similar, except for a predominance of males and differences in living status and patient/maternal education in the DREaM study as compared to the RAISE study (Table 1). Some of these differences may be driven by site differences
The psychiatric history of subjects enrolled in the DREaM and RAISE studies are compared in Table 2. Most characteristics were similar except for the CGI-S rating, suggesting slightly higher disease severity in the DREaM sample at the time of study entry
The median duration of time since the first episode of psychosis (regardless of treatment status) at screening was 11.1 months. In RAISE the median duration of untreated psychosis was approximately 17 months, suggesting that these subjects took longer to begin treatment6
The history of AP exposure and current AP treatment at screening for subjects enrolled in DREaM is shown in Table 3
In comparison, all subjects in RAISE were required to have <6 months of exposure, and 83% were prescribed one or more APs at baseline6
Table 1. Baseline Demographics in the DREaM and RAISE Studies.
| BASELINE CHARACTERISTIC | DREaM: ENROLLED SUBJECTS N = 96 | RAISE: FINAL POPULATION6 N = 404 | P VALUE | ||
| Age, y, mean (SD) | 22.8 (4.0) | 23.1 (5.1) | 0.512 | ||
| Sex, male, n (%) | 83 (86) | 293 (73) | <0.001 | ||
| Race, n (%) | 0.094 | ||||
| White | 47 (49) | 218 (54) | – | ||
| Black | 33 (34) | 152 (38) | – | ||
| Other | 15 (16) | 34 (8) | – | ||
| Missing data | 1 (1) | – | – | ||
| Ethnicity, n (%) | 0.043 | ||||
| Hispanic or Latino | 27 (28) | 73 (18) | – | ||
| Living status, n (%) | <0.001 | ||||
| Independent living | 1 (1) | 72 (18) | – | ||
| Supported or structured | 2 (2) | 14 (3) | – | ||
| With family/friends | 86 (90) | 287 (71) | – | ||
| Homeless/other | 7 (7) | 31 (8) | |||
| Patient education, n (%) | <0.001 | ||||
| Some college or higher | 29 (30) | 125 (31) | – | ||
| Completed high school | 56 (58) | 133 (33) | – | ||
| Some high or grade school | 11 (11) | 125 (31) | – | ||
| No school or unknown | 0 | 21 (5) | – | ||
| Maternal education, n (%) | 0.014 | ||||
| Some college or higher | 49 (51) | 167 (41) | – | ||
| Completed high school | 30 (31) | 111 (27) | – | ||
| Some high or grade school | 13 (14) | 59 (15) | – | ||
| No school or unknown | 4 (4) | 67 (17) | – | ||
Abbreviation: SD, standard deviation.
Table 2. Psychiatric History of Subjects Enrolled in DREaM vs RAISE.
| DREaM: ENROLLED SUBJECTS N = 96 | RAISE: FINAL POPULATION6 N = 404 | P VALUE | |
| Current diagnosis | 0.414 | ||
| Schizophrenia, n (%) | 77 (80) | 214 (53) | – |
| Schizophreniform disorder, n (%) | 19 (20) | 67 (17) | – |
| Other, n (%) | 0 | 123a | – |
| Months since first psychotic episode | |||
| Mean (SD) | 12.2 (7.1) | – | NA |
| Median (range) | 11.1 (2–25) | – | – |
| CGI-S | 0.002 | ||
| Mean (SD) | 4.4 (1.0) | 4.1 (0.8) | – |
| Range | 2–7 | – | – |
| Total number of prior hospitalizations | NA | ||
| Mean (SD) | 1.2 (1.2) | – | – |
| Range | 0–6 | – | – |
| Number of prior hospitalizations, n (%) | 0.145 | ||
| 0 | 28 (29) | 88 (22) | – |
| 1 | 33 (34) | 181 (45) | – |
| 2 | 21 (22) | 69 (17) | – |
| ≥3 | 12 (13) | 64 (16) | – |
| Missing data | 2 (2) | 2 (0.5) | – |
| Cumulative substance history, n (%) | NA | ||
| <6 months | 2 (2) | – | – |
| 6–12 months | 1 (1) | – | – |
| >12 months | 59 (61) | – | – |
| Missing data, n (%) | 34 (35) | – | – |
Notes: aA total of 123 subjects in the RAISE study had psychotic diagnoses other than schizophrenia or schizophreniform disorder.
Abbreviations: NA, not applicable.
Table 3. AP Treatment at Screening for DREaM Subjects.
| DREaM SUBJECTS N = 96 | |
| Duration of prior AP exposure, n (%) | |
| <6 months | 48 (50) |
| 6–12 months | 16 (17) |
| >12 months | 23 (24) |
| Missing data, n (%) | 9 (9) |
| Current AP treatment at screening,a n (%) | |
| Risperidone | 25 (26) |
| Paliperidone | 18 (19) |
| Olanzapine | 12 (13) |
| Haloperidol | 8 (8) |
| Quetiapine | 8 (8) |
| Aripiprazole | 6 (6) |
| Lurasidone hydrochloride | 3 (3) |
| Other | 3 (3) |
| Loxapine | 1 (1) |
| Ziprasidone hydrochloride | 1 (1) |
| Not assignedb | 22 (23) |
Notes: aDrugs selected if they are used on the reference start date or 2 weeks before it.
bSubjects who were not assigned to any medications.
Additional Clinical Characteristics of DREaM
Most subjects enrolled in DREaM (80%) had a CGI-S score of ≥4, which is indicative of a population that is moderately to extremely ill (Figure 2)
At baseline, there was a wide range of satisfaction with current AP medication, with the most common response being “somewhat satisfied” (27%; Figure 3)
The mean ± SD MCCB score is 28.2 ± 13.9; 42% of subjects had a score of ≤25 (Figure 4)
The screening mean ± SD PSP scale score is 49.5 ± 14.6 (range, 5–80). Most subjects had a score of 31 to 70 (86%), indicating a moderate degree of social dysfunction at baseline (Figure 5)
The most severe CRDPSS ratings were observed in delusions, negative symptoms, and impaired cognition (Figure 6)
Figure 2.

Baseline CGI-S Scale
Figure 3.
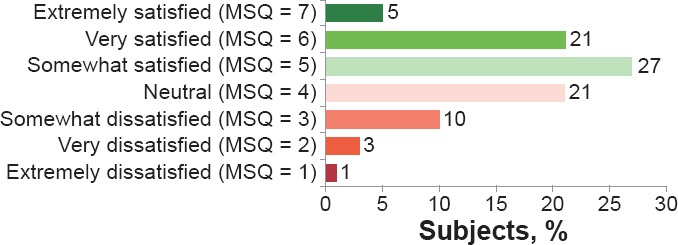
Medical Satisfaction as Assessed Using the MSQ at Screening (N = 96)
Figure 4.
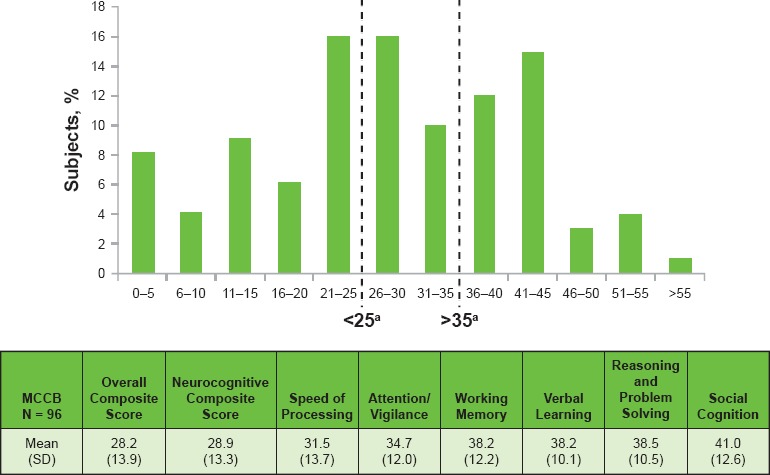
Cognition as Assessed Using the MCCB at Screening (N = 93)
Figure 5.

Baseline PSP Scale Total Scores (A) and Domain Scores (B)
Figure 6.
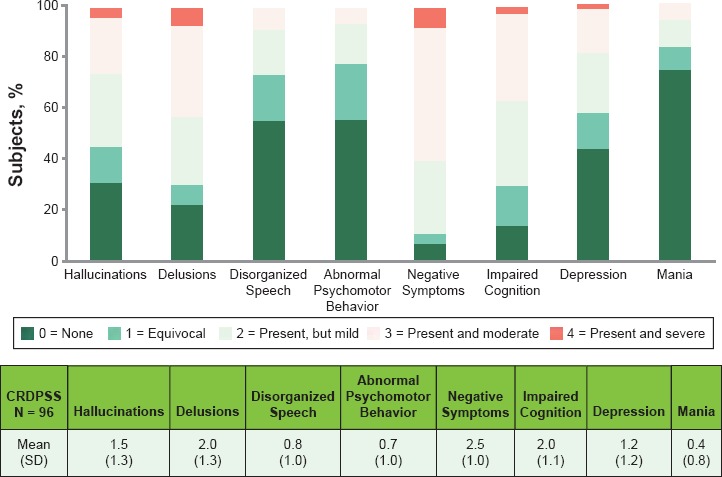
Frequency of CRDPSS Item Ratings at Screening
Limitations
Between the two studies, there were differences in both inclusion criteria and research site types
The DREaM study sample presented here represents only one-third of the planned enrollment. Thus, it may not reflect the full population to be studied
Discussion
Most subjects with recent-onset psychosis enrolled in the DREaM study had at least one prior hospitalization, a history of substance use, a CGI-S score of ≥4, and a moderate degree of social dysfunction
With a few exceptions, the baseline demographics and characteristics of subjects in the DREaM study appear similar to those reported in the RAISE study in subjects with first-episode psychosis receiving treatment at US community mental health centers
The DREaM study population’s baseline characteristics and clinical data represent those of a population recently diagnosed with schizophrenia and will be used to match subjects in anticipation of randomization for Parts 2 and 3 of the study
Acknowledgments
The authors thank Matthew Grzywacz, PhD, and Lynn Brown, PhD, of ApotheCom (Yardley, PA) for their writing and editorial assistance.
Footnotes
Presented at the American Psychiatric Association Annual Meeting, May 20–24, 2017, San Diego, CA.
Disclosures
B. Brown, L. Alphs, and Y. Yue are employees of Janssen Scientific Affairs, LLC, and are Johnson & Johnson stockholders. I. Turkoz is an employee of Janssen Research and Development, LLC, and is a Johnson & Johnson stockholder.
References
- 1.Harrison G et al. Br J Psychiatry. 2001;178:506–517. doi: 10.1192/bjp.178.6.506. [DOI] [PubMed] [Google Scholar]
- 2.Nuechterlein KH et al. J Clin Psychiatry. 2014;75:25–29. doi: 10.4088/JCP.13065.su1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subotnik KL et al. JAMA Psychiatry. 2015;72:822–829. doi: 10.1001/jamapsychiatry.2015.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartzokis G et al. Schizophr Res. 2012;140:122–128. doi: 10.1016/j.schres.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Agostino RB. N Engl J Med. 2009;361:1304–1306. doi: 10.1056/NEJMsm0904209. [DOI] [PubMed] [Google Scholar]
- 6.Kane JM et al. Am J Psychiatry. 2016;173:362–372. doi: 10.1176/appi.ajp.2015.15050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuechterlein KH et al. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 8.Nasrallah H et al. Psychiatry Res. 2008;161:213–224. doi: 10.1016/j.psychres.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976. Revised, 1976. [Google Scholar]
- 10.Kalali A. Curr Med Res Opin. 1999;15:135–137. doi: 10.1185/03007999909113374. [DOI] [PubMed] [Google Scholar]
- 11.Kalali AH. Clear Perspectives. 1999;2:18–21. [Google Scholar]
- 12.Heckers S et al. Schizophr Res. 2013;150:11–14. doi: 10.1016/j.schres.2013.04.039. [DOI] [PubMed] [Google Scholar]


