Figure 1.
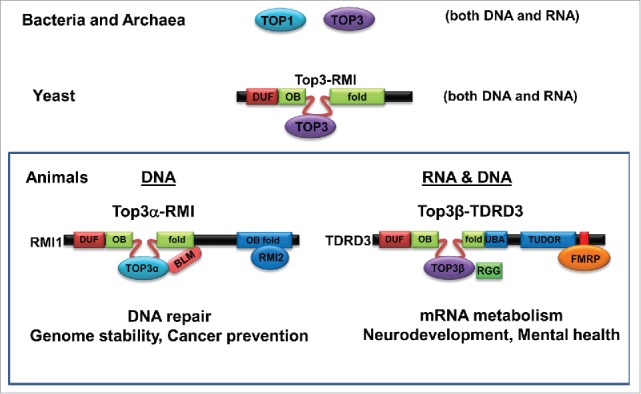
Type IA topoisomerases have evolved from single proteins with dual activities in microorganisms to multi-protein complexes with restricted activities in animals. Schematic representation of evolution of Type IA topoisomerases in DNA and RNA metabolism. In E. coli, both Type IA enzymes (Top1 and Top3) have dual activities for DNA and RNA. In yeast, the only Type IA enzyme is part of a complex (Top3-Rmi1) that also has dual activities for DNA and RNA. In animals, only one of the 2 Type IA paralogs, Top3β, has dual activity, whereas Top3α has activity for only DNA. Interestingly, Top3β, but not Top3α, contains an RNA-binding domain (RGG box) that is required for its RNA topoisomerase activity. Moreover, the 2 Top3 paralogs comprise 2 distinct complexes, with the Top3β complex containing a RNA binding protein (FMRP), whereas the Top3α complex containing a DNA helicase (BLM). These data argue that Type IA topoisomerases have evolved into 2 functional distinct complexes in animals, one for RNA and DNA (Top3β-TDRD3-FMRP), and one for DNA only (Top3α-Rmi1-Rmi2-BLM). This figure is adapted from Fig. 7A of a previous publication.28
