Abstract
Numerous factors including chemical, hormonal, spatial and physical cues determine stem cell fate. While the regulation of stem cell differentiation by soluble factors is well characterized, the role of mechanical force in the determination of lineage fate is just beginning to be understood. Investigation of the role of force on cell function has largely focused on “outside-in” signaling, initiated at the plasma membrane. When interfaced with the extracellular matrix, the cell utilizes integral membrane proteins, such as those found in focal adhesion complexes to translate force into biochemical signals. Akin to these “outside-in” connections, the internal cytoskeleton is physically linked to the nucleus, via proteins that span the nuclear membrane. Although structurally and biochemically distinct, these two forms of mechanical coupling influence stem cell lineage fate and, when disrupted, often lead to disease. Here we provide an overview of how mechanical coupling occurs at the plasma and nuclear membranes. We also discuss the role of force on stem cell differentiation, with focus on the bio-chemical signals generated at the cell membrane and the nucleus and how those signals influence various diseases. While the interaction of stem cells with their physical environment and how they respond to force is complex, an understanding of the mechanical regulation of these cells is critical in the design of novel therapeutics to combat diseases associated with aging, cancer, and osteoporosis.
Keywords: LINC, emerin, nesprin, lamin, vibration, exercise, mesenchymal stem cell
Introduction
Biological systems are fine-tuned to sense, respond, and adapt to physical stimuli [1]. In the case of stem cells, the external physical environment guides stem cell fate decisions [2]. As such, stem cells, as a prototype of multiple descendent lineages, possess mechanosensory machinery to translate mechanical signals into biochemical responses. Further, cells adapt and respond to physical forces by remodeling their internal physical structures to regulate interactions with the external physical environment. Application of mechanical force initiates signaling cascades at the plasma membrane leading to the generation and remodeling of filamentous actin stress fibers, which enhance the cell’s ability to perceive and transmit mechanical force intracellularly [3].
For bone marrow mesenchymal stem cells (MSCs), a more structured cytoskeleton leads to differentiation into lineages making up tissues with greater mechanical competence (i.e., bone, cartilage). It is important to note that MSCs form heterogeneous populations, distributed along a dynamic differentiation spectrum. While the question of whether the contribution of MSCs to different lineages stem from direct fate switching or a modular responsiveness of different sub-populations remains unresolved, it is reasonable to expect that up until some point the fate of MSC populations remain plastic, rendering them susceptible to incoming mechanical signals [4]. In this way, both “outside-in” and “inside-out” force signaling determine MSC lineage fate. In the former, the stiffness of the substrate [5], or application of external mechanical force [6], induces intracellular effects. In inside-out signaling, scaffolding of intracellular proteins reinforces integrin-based attachments [7].
At the interface between the basal substrate and adjacent cells, the plasma membrane utilizes specific structural proteins to transduce force into biochemical signals. These structural proteins gather together to form focal adhesions (FAs), which span the cell membrane and connect the extracellular matrix to the actin cytoskeleton. Focal adhesions also serve as hubs for signaling molecules to congregate, where the combination of force transmission and signal transduction result in further cytoskeletal remodeling [8]. Recent work also identifies the nucleus, and its membrane, as mechanosensory organelles, where anchoring to the cytoskeleton via the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex enables transmission of mechanical force between the nucleus, the cytoskeleton outward to the external microenvironment [9]. In this way, mechanical signals have the potential to further regulate connections between the nucleus and the cell cytoskeleton, generating another level whereby mechanical input can control cell behavior. Thus, while it has become accepted that genetic elements within the nucleus respond to mechanical challenges indirectly through their transduction into intermediary biochemical cascades [1], it has only recently been considered that applied forces might also directly alter chromosomal conformations, thus influencing the accessibility of genetic information for binding of transcriptional enhancers or repressors [10].
As mechanical signals are critical for directing cellular responses, dysfunction of the mechanosensory machinery can lead to disease. One such example at the level of nuclear force control is Hutchinson-Gilford progeria syndrome, which manifests symptoms of aging at a very early age. This condition arises due to due a mutation in Lamin A/C, a structural protein at the inner nuclear membrane [11]. Thus, unlike diseases of “accelerated aging” including Werner syndrome, which are caused by defective DNA repair, this “laminopathy” results from inadequate mechanical support and abnormal nuclear structure [12]. Thus, the structural relationship of Lamin A/C and other nuclear membrane scaffolding proteins, represents an interesting target to combat the degenerative effects of conditions associated with disuse and aging, especially since the structural relationship of these nuclear membrane scaffolds affects the accessibility and action of transcriptional regulators.
In this manuscript we focus on the example of the influence of mechanical force on bone marrow mesenchymal stem cell differentiation, especially as it relates to the balance of bone and fat formation. Stem cells in other tissues, subject to unique mechanical environments, will have variations on this theme. The consequences of reduced mechanical loading on bone marrow MSC lineage allocation will be discussed, as will the role of various mechanosensory elements, with an emphasis on the contrasting role of force transduction at the plasma membrane with that at the nuclear envelope. Additionally, we will consider the role of mechanobiology in human disease and explore how disrupted mechanocoupling at differing subcellular locations can lead to pathology.
Cellular architecture influences MSC differentiation
Mesenchymal stem cells are abundantly found within the bone marrow and adipose depots and have the ability to develop into mesenchymal tissues including bone, cartilage, fat and muscle [13]. MSC lineage fate is strongly dependent upon the context of the physical environment, both topographical and mechanical qualities of the surrounding matrix, as well as the dynamic mechanical environment modulate the allocation of MSCs [1].
Cellular structure is largely determined by its cytoskeleton, which is a dynamic structure primarily composed of three types of proteins: microfilaments (actin), microtubules (tubulin), and intermediate filaments [14]. While actin fibers and microtubules are made up of one type of monomer (actin and tubulin respectively), intermediate filaments have a wide variety of different monomers that form the larger filamentous structures. Hundreds of other proteins associate with the cytoskeleton including crosslinkers, molecular motors, and signaling effectors. Cytoskeletal fibers, especially actin filaments, are acutely sensitive to both chemical and physical modulation. For instance application of mechanical force enables globular actin monomers (G-actin) to aggregate and form structured filamentous fibers (F-actin). Formation of actin fibers, from monomers, is controlled in large part by Rho GTPases including RhoA, where exchange of GTP induces activation of RhoA, leading to actin stress fiber polymerization [15]. Thus, the structure of the cytoskeleton is extremely dynamic, enabling modification of cellular shape, intracellular signaling events, and thus cellular behavior and phenotype.
Mechanically, cell structure undergoes a continual balance of inner and outer forces which allow cellular components to experience both compression and tension, a process summed in the concept of tensegrity [16]. The tensegrity structure acts as a global sensory mechanism for mechanical cues, allowing for rapid structural responses to static and dynamic mechanical loads [17]. Such structural responses are mediated through the actin cytoskeleton, where focal adhesion (FA) connections facilitate the tensegrity balance, connecting the inner cytoskeleton with the matrix outside the cell.
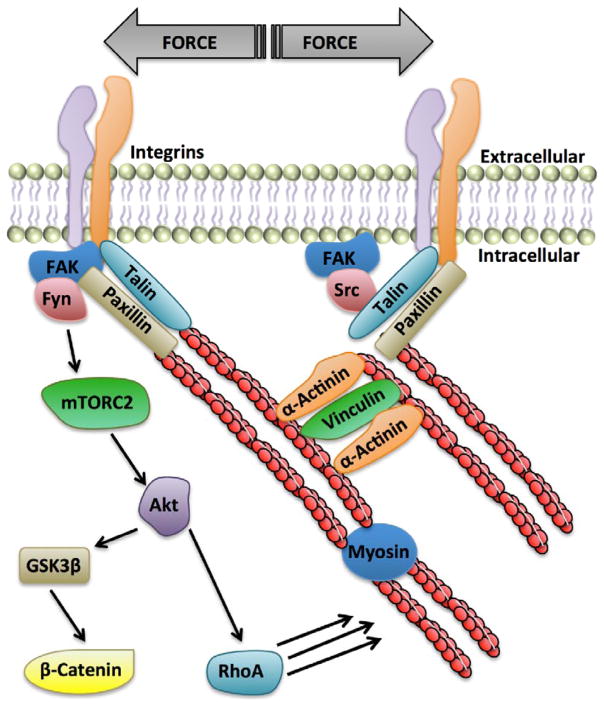
Early studies showed that increased matrix stiffness enhanced the size of FA sites in fibroblasts, while softer substrates resulted in smaller, more mobile FAs [18]. As FAs are the physical “bridge” between the actin cytoskeleton and the extracellular matrix (Fig. 1), these studies highlight the ability of the matrix to signal internally, resulting in adaptation to the mechanical forces. Additional work using MSCs demonstrated that confining cell adhesion by plating cells on predetermined fibronectin substrate islands was able to control subsequent cell shape and the level of actomyosin contractility by regulating RhoA/ROCK signaling [19]. MSCs forced to attach on smaller fibronectin islands assumed a rounder shape, consistent with decreased cell stiffness, and showed increased adipogenic differentiation. In contrast, attachment to larger island areas resulted in greater cell spreading, increased RhoA/ROCK activity, and favored osteogenic lineage. Similarly, matrix elasticity regulates the internal cell tension and differentiation of MSCs. Soft substrates (0.1–10kPa) induce neuronal and adipogenic differentiation [20, 21], while substrates with stiffness comparable to muscle tissue (~10–17kPa) supports allocation to myocytes [22]. Even stiffer substrates (>25kPa), akin to hard bone surfaces, promote osteogenic differentiation [23]. These studies highlight the influence of RhoA/ROCK pathway on cellular architecture, confirming that cellular tension generated by an enhanced cytoskeleton informs MSC lineage fate.
Fig. 1.
Force transmission across the plasma membrane employs membrane-spanning integrins, which connect to the actin cytoskeleton via talin and paxillin linker molecules. These focal adhesion sites also serve as signaling hubs for mechanosensitive kinases such as Fyn and FAK, which restrict MSC adipogenesis by activating Akt which both enhances β-catenin availability and increases RhoA-mediated cytoskeletal assembly.
How might cellular architecture regulate lineage decisions? We are now learning that changes in cytoskeletal architecture not only modulate the physical qualities of the cell but also direct the reconfiguration of the mechanosensory signaling mechanisms within the cells. Yorkie-homologues YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif) are examples of transcription factors that regulate MSC proliferation and differentiation [24] in response to mechanical cues [25]. Impaired YAP/TAZ activity was found when MSCs grown on softer matrices and following disruption of the actin cytoskeleton, by blocking RhoA activity [25]. These data indicate that MSC fate selection towards osteogenic or adipogenic lineages is modulated by YAP/TAZ, whose activity is modulated by both the mechanical properties of the extracellular matrix and the stiffness of the internal actin cytoskeleton. Furthermore, knock down of YAP/TAZ resulted in loss of mechanical regulation of MSC differentiation, where depletion of YAP/TAZ not only inhibited osteoblastic differentiation but also promoted adipogenesis [26]. Similarly, adding adipogenic differentiation media down regulated RhoA/ROCK activity, leading to an increased monomeric G-actin to polymerized filamentous-actin (F-actin) ratio. This relative increase in monomeric actin is presumably the result of depolymerization of F-actin, which indirectly results in export of the transcriptional coactivator MKL1 (megakaryoblastic leukemia 1, MAL) from the nuclear compartment [27]. Furthermore, the increase in cytoplasmic G-actin traps MKL1 outside the nucleus, releasing peroxisome proliferator-activated receptor gamma (PPARγ) repression, with consequent stimulation of adipogenic pathways. Actin dynamics also regulate the activity of the Serum Response Factor (SRF), allowing this molecule to shuttle into the nucleus [28] where it interacts with MKL1, whose function is specifically dependent on intranuclear actin polymerization [29]. While F-actin assembly within the nucleus controls MKL1 function, the responses of F-actin structures to chemical (i.e., serum) and physical (i.e., integrin engagement) stimuli remain poorly understood [30].
To further highlight the importance of actin dynamics to MSC fate, Sen and colleagues recently used cytochalasin D, a mycotoxin that depolymerizes filamentous actin struts (F-actin) into globular-actin (G-actin) monomers. Reducing actin fibers to their monomeric components resulted in mass import of actin into the nucleus, and once inside the nucleus, actin monomers facilitated export of YAP, which normally suppresses Runt-related transcription factor 2 (Runx2) activity. The release of Runx2 repression contributes to accelerated osteogenesis [31]. These data suggest that the idea that F-actin depolymerization leads to adipogenesis, a dog-matic view in the field of MSC biology, should be amended. While formation of F-actin stress fibers pushes MSCs towards the osteogenic lineage by increasing the internal physical tension, full depolymerization of actin polymers leads to translocation of actin monomers into the nucleus. Localization of G-actin in the nucleus promotes osteogenic gene transcription, perhaps representing a secondary level of actin structural control within the nucleus itself. Importantly, injection of cytochalasin D into the tibial marrow space of live mice results in abundant bone formation [31]. Thus, both the structural filamentous actin fibers and the monomeric G-actin components have critical roles in regulating MSC lineage allocation, and their location in the cytoplasm or within the nucleus might provide differential control of transcriptional processes.
Mechanical force transmission at the plasma membrane
There are a variety of different mechanosensory structures capable of conveying mechanical information across the cell membrane including changes in lipid microdomains [32], G-protein coupled receptors [33], mechanosensitive ion channels [34, 35], and focal adhesion-based integrin attachments [36]. Integrins are perhaps the most widely recognized membrane-associated mechanosensory molecules. These heterodimeric molecules span the plasma membrane and use linker proteins, including talin [37] and vinculin [38], to attach to the internal actin cytoskeleton. Externally, connection of the actin stress fibers to the extracellular matrix (ECM) enables a functional attachment through which force can be distributed across the cell membrane to the matrix substrate (Fig. 1).
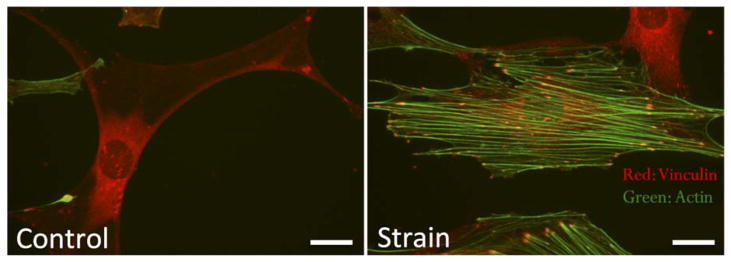
The physical attributes (i.e., stiffness, topography, etc.) of the ECM and the type of mechanical signals incurred (i.e., strain, fluid shear stress, etc.) influence integrin-mediated force transmission. A stiffer substrate induces osteogenic differentiation; whereas, a less stiff substrate leads to adipogenic lineage fate [5]. Similar changes in MSC differentiation can be achieved by applying dynamic mechanical loads. Externally applied substrate strain leads to an increase in FA formation and maturity of bone marrow MSCs [3]. The increase in matrix attachment contact points is correlated with increased actin stress fiber formation, but also influences intracellular signaling. Interestingly, MSCs exposed to a twenty-minute bout of mechanical strain (200 cycles at 2% deformation) resulted in a greater reduction of adipogenic commitment than 3,400 continuous strain cycles under the same deformation parameters [6]. Furthermore, providing a second bout of strain, following a 3-hour rest period, induced greater activation of anti-adipogenic signals, suggesting that the initial application of force induces a priming effect, whereby intracellular signals are then positioned to react to the subsequent mechanical challenge.
The ability of mechanical signaling pathways to be amplified, depending on the timing of the mechanical regimen, demonstrates that FAs serve as signaling platforms. As such, applied forces induce recruitment of signaling effectors to FAs, where they are positioned to acutely respond to the next loading event (Fig. 2). It has been well recognized that FAs serve as signaling hubs in many cell types, with molecules such as the Src family tyrosine kinases, extracellular signal-regulated-kinase (ERK), and focal adhesion kinase (FAK) functioning in association with FAs [39]. Following application of mechanical force, FAK is one of the first signals to arrive at the maturing FA, which is soon followed by Src. These kinases then induce activation of downstream signals including the growth-factor-receptor-bound protein 2 (GRB2) adaptor protein, Ras, and ERK [40].
Fig. 2.
Substrate strain (2%, 100 cycles) induces cytoskeletal reorganization with a 5-fold increase in focal adhesions (vinculin, red) and F-actin (green). Scale bars = 25 mm.
In bone marrow MSCs, integrin-initiated signals are the genesis of a cascade that restricts adipogenic line-age fate in response to mechanical force. While some of the molecular mechanisms are conserved in the multiple cell types, MSCs appear to utilize several unique signaling mechanisms to respond to mechanical challenges. For example, mechanical activation of Src and FAK occur readily in fibroblasts [39]; however, in place of Src, MSCs recruit the Src-family member Fyn to FAs. Once located in the FA complex, Src and FAK cooperate to induce Akt phosphorylation at serine 473 (S473) via mammalian target of rapamycin 2 (mTORC2)[41]. ERK1/2 activation is another mechanically activated pathway in fibroblasts [39]. Some studies suggest that ERK signaling is important for mechanical control of MSC differentiation, by activating the osteogenic transcription factor Runx2 [42], while others indicate that ERK1/2 signaling is not involved in force-induced MSC lineage fate decisions that prevent adipogenesis [41]. These contrasting results may be due to different types of force applied to the cells, or simply convey the idea that there are multiple signaling pathways emanating from the FA complexes that are arbitrarily measured in response to time or input.
Mechanical activation of mTORC2, by Fyn and FAK, results in adipogenic repression of MSCs by acting through two distinct, but complementary pathways, one altering transcriptional regulation of adipogenic genes, while the other enhances cytoskeletal structure resulting in a more rigid cell. In both cases, recruitment of Fyn and FAK to focal adhesions results in activation of mTORC2, which in turn phosphorylates Akt at S473 [41, 43]. To regulate gene expression, Akt phosphorylates GSK3β at serine-9, resulting in GSK3β inactivation thus preventing proteasomal degradation of βcatenin (Fig. 1). Nuclear translocation of βcatenin then presumably enables transcriptional modification of LEF and TCF targeted transcription factors, or by directly repressing PPARγ activity, resulting in down regulation of adipogenic genes [44].
While control of βcatenin activity directly regulates adipogenic gene expression, mechanical activation of mTORC2 also impacts MSC lineage by enhancing formation of actin stress fibers. Similar to the way that a stiff substrate drives MSCs towards osteogenesis, by altering the physical environment of the cell, mechanical strain generates enhanced internal tension by formation of a filamentous actin network. Assembly of these actin fibers requires activity of RhoA, a GTPase that is acutely responsive to mechanical force. In MSCs, RhoA activation occurs after less than 100 cycles of 2% membrane deformation, followed by stress fiber assembly [3]. Applied substrate force is sensed at focal adhesion interfaces, where Fyn and FAK are recruited to the existing FA complex. Similar to the pathway resulting in preservation of βcatenin, Fyn activates mTORC2, resulting in Akt phosphorylation [41]. Recent work has shown that RhoA requires mechanically activated Akt to induce cytoskeletal remodeling and restrict entry of MSCs into the adipogenic lineage [41]. Thus, mechanical force leads to anti-adipogenic pathways that begin at the FA and diverge to regulate both nuclear βcatenin localization and formation of actin stress fibers, through RhoA (Fig. 1).
While integrin-mediated attachments at focal adhesions are a primary site of signal initiation to direct mechanical control of MSC fate, primary cilia provide another unique mechanosensory apparatus capable of influencing MSC differentiation. Each cell contains only one cilium, a non-motile microtubule-based organelle emerging from the distal centriole of the centrosome of many mammalian cells [45]. In contrast to the numerous and continually remodeling FAs needed to convey mechanical force to the cell, only a single hair-like cilia structure is required to induce biological responses. A compelling body of literature has shown that these structures mediate critical mechanosensory effects in various types of bone cells [46], including mesenchymal bone progenitors [47]. Knockdown of proteins that form the primary cilium in human MSCs results in repression of both Runx2 and PPARγ expression, suggesting that primary cilia also regulate osteogenic and adipogenic differentiation [48]. Additional work has recently shown that MSCs lacking primary cilia are deficient in several mechanosensory responses [49].
Nucleus as a force-responsive organelle
The nucleus is poised to recognize physical signals from the environment as it is mechanically integrated into the cell structure through proteins that span the nuclear envelope enabling connections between intranuclear structures and the intracellular cytoskeleton. These nuclear to cytoplasmic connections not only provide spatial and structural integrity, but also serve to transfer mechanical force across the nuclear envelope [50]. It is likely that the nucleoskeleton, and tension associated externally and internally, alter the structure of chromatin interfaces with the inner nuclear envelope and influence gene expression.
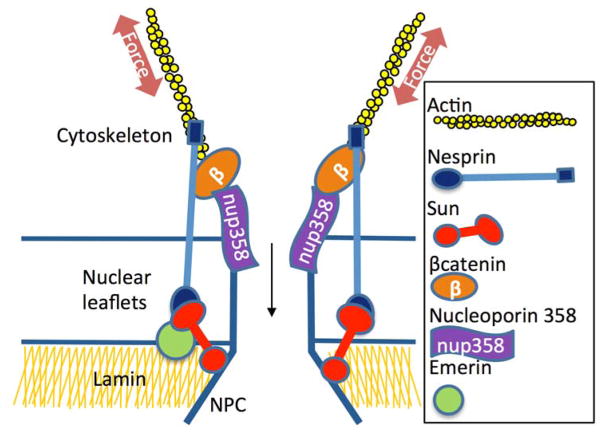
Lamin A/C scaffolding proteins inside the nuclear envelope are mechanically coupled to the cytoplasmic cytoskeletal elements via LINC complex proteins. A key LINC protein group providing actin connectivity are the actin-binding giant Nesprin isoforms of the spectrin repeat protein that spans the nuclear envelope. By utilizing N and C terminal domains located at opposite ends of the protein, Nesprin attaches to actin fibers located in the cytoplasm via the calponin homology (CH) domain at the N-termini [51, 52] while the Klarsicht, ANC-1, Syne Homology (KASH) domain at the C-termini binds to SUN proteins on the inner nuclear leaf-let [53]. As such, Nesprin is part of a structural scaffold that connects the nucleus to the actin cytoskeleton providing a continuous connection that perceives and adapts to mechanical challenges [54, 55]. The ability of force to be transferred between actin fibers and the nucleus is evidenced by the development of organized structures, called transmembrane actin-associated nuclear (TAN) lines [56] (also called the “actin cap”), which develop when cytoskeletal stress is increased [57]. Such nucleo-cytoskeletal connectivity not only provides a framework by which the nucleus is attached to the cytoskeleton, but the connectivity can itself adapt. As such, forces applied to the nucleus via the cytoskeleton will recruit LINC complex proteins and Lamin A/C to specific locales under stress fibers that press on the nuclear envelope [58]. The local accumulation of LINC proteins in response to local forces along the nuclear membrane is analogous to the dynamic maturation and proliferation of FAs on the plasma membrane in response to mechanical input at substrate attachment sites. Interestingly, the nuclear envelope, and by extension LINC complexes at the nuclear envelope, have been implicated as nucleation points for actin polymerization at the cytoplasmic side of the nucleus [59]. This suggests that, just like focal adhesions, initiation of actin polymerization at the nuclear envelope can attract other scaffolding proteins such as α-actinin [60] and zyxin [61]. This leads us to propose that LINC complexes are the nuclear equivalent of focal adhesions. While force induces recruitment of proteins including talin, paxillin, and vinculin to form focal adhesions at the plasma membrane, mechanical force similarly recruits Nesprin, SUN, and lamin proteins to create focal contacts at the nuclear membrane. As such, focal attachments, associated with the nuclear envelope play a direct mechanosensory role by enabling the transmission of externally or internally generated mechanical signals between nucleus and the actin cytoskeleton [54, 62, 63]. At this juncture, LINC complexes are being recognized for their contribution to the mechanosensory function of cells [64, 65] – including the sensation of sound [66] and mechanical vibrations [63]–but the mechanisms by which they regulate lineage allocation of MSCs remains unclear.
How might the changes in nuclear structure and connectivity modulate MSC mechanoresponses and differentiation? Recent evidence suggests that, similar to extracellular matrix stiffness, application of substrate strain increases the nuclear translocation of YAP [67]. This response is dependent on the level of cytoskeletal pre-stress and nuclear deformation, but critically requires the structural connection between Nesprin-1 and actin; depletion of Nesprin results in greatly diminished nuclear YAP translocation [68]. These data suggest that nuclear transport of YAP requires Nesprin-mediated connections to the cytoskeleton, and this response is dependent on mechanical force. Other nuclear transport processes have been linked to Nesprin including that of βcatenin [69].
Another way in which mechanical force might regulate MSC fate via nuclear envelope connections is through LINC attachment to the inner nuclear Lamin A/C network. Lamin A/C binds to SUN proteins forming a scaffold on the inner leaflet of the nucleus [70]. In support of the role of LINC/nucleoskeleton control, adipogenic differentiation is associated with a decrease in Lamin A/C expression [71], and both partial or complete deletion of Lamin A/C accelerates adipogenic differentiation in MSCs [72–74]. In contrast, osteogenic differentiation leads to increased Lamin A/C expression [75], a change consistent with the increased cellular stiffness of osteoblasts [76, 77]. Additionally, overexpression of Lamin A/C promotes osteogenic differentiation [78]. These observations support the idea that Lamin A/C is associated with MSC lineage decisions, but it remains unclear whether the changes in Lamin A/C are the cause or effect of differentiation. As cells deficient in Lamin A/C and LINC complex proteins are more susceptible to mechanical damage [23, 79], it is intriguing to postulate that MSCs require full function of these structural proteins to properly respond to physiological forces and adapt to a changing mechanical microenvironment.
Direct evidence for LINC-mediated force transmission and regulation of nuclear stiffness has been demonstrated recently by Guilluy and colleagues [80], where Nesprin-bound bead motion by magnetic force caused stiffening of an isolated nucleus through phosphorylation of the LINC binding partner Emerin in a Src and Lamin A/C-dependent manner. This suggests that mechanical signals directly modulate the nucleoskeletal structure and in turn might influence MSC lineage fate. Recent work completed using multiple cell lines, demonstrated that Lamin A/C polarization within the nucleus is controlled by cytoskeletal force, and depletion of cytoskeletal tension disrupted this Lamin A/C polarization [81]. Conversely, deletion or mutation of Lamin A/C alters mechanical actin dynamics and interferes with MKL1-mediated serum response factor (SRF) activity [82]. Interestingly, MKL1 nuclear localization in Lamin A/C deficient cells was primarily dependent on the localization of Emerin at the nuclear envelope rather than on LINC-mediated nucleo-cytoskeletal connectivity [82]. This suggests that Emerin might also affect intranuclear actin dynamics to regulate MKL1 localization and functionality [83].
Consistent with the hypothesis that force can rear-range nucleoskeletal structure to modulate MSC fate, our lab and others, have shown that repeated application of mechanical input, such as substrate strain or high frequency vibration, sensitizes the cell to subsequent mechanical stimuli [84, 85]. As discussed above, this increased sensitivity to very low intensity mechanical signals is in part due to augmentation of focal adhesions and RhoA signaling [3, 41]. Furthermore, increased RhoA activity has been correlated to enhanced force transfer into the nucleus [86]. Additional evidence demonstrates that the physical connections between the nucleus and cytoskeleton are capable of altering the structure and position of nuclear proteins. Recent findings show that the dynamic force application upon integrins, via substrate strain, directly controls the displacements of coilin and SMN proteins in Cajal bodies within the nucleus. Displacement of these proteins within the Cajal body nuclear sub-organelles was directly proportional to the magnitude of the strain and was dependent on both cytoskeletal stress and the presence of Lamin A/C [87]. These findings support the idea that the nucleus participates directly in sensing forces from outside the cell. Thus it is likely that LINC-mediated mechanical coupling, between the nucleus and the cytoskeleton, impacts nuclear structure and function. In fact, we have shown that the LINC complex acts as a primary mechanosensory element that senses and initiates signaling in response to low intensity vibration, while substrate strain can initiate signaling independently of LINC connectivity [88]. As such, both low and high intensity mechanical forces, through the tensegrity continuum, are eventually transferred throughout the cell. With this in mind, it will be important to consider that alterations in nuclear-cytoskeletal connections may contribute to the decreased perception of mechanical input associated with aging, microgravity, or other musculoskeletal conditions. As such, a loss in LINC complex-dependent mechanosensitivity might even contribute to reported failure of musculoskeletal tissues by limiting the accessible spectrum of mechanical information.
Dysfunctional mechanobiology
The nuclear envelope has been termed “the most important border in the eukaryotic cell” [75]. Mutations involving Lamin A/C, LINC complex proteins, and their binding partners are associated with a variety of musculoskeletal conditions, including Hutchinson-Gilford progeria [89], Emery-Dreifuss muscular dystrophy [90], and dilated cardiomyopathy [91]. Nuclear envelopathies in humans and mice are characterized by failure of mechanoresponsive tissues, including bone, skeletal muscle, and heart; all tissues of mesenchymal origin. Transgenic mice with alterations in Lamin A have progeria with cardiomyopathy and sarcopenia, exhibit low bone mass, and have increased bone marrow adiposity [74]. Our group previously established that mechanical activation of βcatenin preserves MSC multipotentiality [92] and daily mechanical input effectively counteracts adipogenic stimuli, disrupting adipogenesis of MSCs [93, 94]. MSC lineage allocation, as noted above, is strongly influenced by nucleoskeletal structure and LINC function. The contribution of LINC complexes may be in regulating βcatenin compartmentalization, as nuclear translocation of βcatenin first requires binding to Nesprin on the outer nuclear membrane [69]. Indeed, limiting LINC function leads to accelerated MSC adipogenesis [88], a phenotype suggested in lamin A-deficient mice, which have musculoskeletal defects, fatty infiltration in the bone marrow, along with decreased βcatenin signaling [90]. In contrast, Emerin, another binding partner of the LINC complex [95], controls nuclear export of βcatenin; depletion of Emerin preserves nuclear βcatenin, thus limiting MSC adipogenesis [96]. Although the exact mechanisms through which LINC complexes control intracellular βcatenin compartmentalization remain poorly understood, it is known that βcatenin requires direct contact with the nuclear pore complex (NPC) for entry into the nucleus [97]. Furthermore, disruption of the nuclear pore protein, Nucleoporin 358 (Nup358), results in suppression of rapid nuclear βcatenin import [98]. As Nesprin forms a tight association with βcatenin [69], it is interesting to speculate that Nesprin, or other LINC proteins, interact with the NPC to regulate βcatenin availability and therein, MSC fate decisions (Fig. 3).
Fig. 3.
Model of LINC-mediated βcatenin availability in the vicinity of Nuclear Pore Complex (NPC). F-actin cytoskeleton associates with the calponin domain of Nesprin, which connects to Sun proteins through the KASH domain. Sun 1 and 2 link to the inner nuclear LaminA/C cytoskeleton. Known association between Nesprin and βcatenin may increase access of βCatenin to Nucleoporin Nup358, which facilitates nuclear import of βCatenin.
Dysregulation of Lamin A/C and the LINC complex each play an important role in aging. During the aging process, the Lamin A/C network is diminished [75] and mutated forms of Lamin A/C arise more frequently [99]. Mutations in Lamin A/C are detrimental to the formation and maintenance of LINC/nucleoskeleton connections [100], and may contribute to age-related cell senescence [101]. In mice, aging not only results in decreased Lamin A/C expression [102] but partial knock-down of lamin A/C (Lmna+/−) is associated with increased fat infiltration as well as impaired bone and muscle function [73]. Importantly, Lamin A/C mutant mice do not form bone in response to exercise, but instead lose bone, resulting in fractures [103].This suggests that physical connections between the cell cytoskeleton and the nucleus are important in MSC differentiation and are critical for mechanical competence of the cell. As such, one might propose that failure of musculoskeletal tissues during aging reflects an insufficient MSC response to the experienced mechanical environment; i.e., an inability of MSCs to repair and reorganize those tissues subject to mechanical stress.
Outlook & Conclusions
In the last decade considerable progress has been made in identifying the mechanisms by which cells sense and respond to both static and dynamic mechanical cues by initiating signaling events, and remodeling cellular architecture. Mechanically induced structural changes modulate transmission of force within cells. Moreover, sensation of the mechanical qualities of the environment is critical in directing cellular function and, in the case of MSCs, regulating lineage selection. In this way, cells maintain a continuous flow of information - both mechanical and biochemical - between the nucleus, the cytoskeleton, and the outside environment. The importance of the physical connections between the nucleus and the cellular cytoskeleton have become increasingly apparent in recent years, especially in light of the propensity for disease accompanying the loss of structures such as Nesprin or the Lamin A/C network. As stem cells rely on mechanical cues from the environment to reorganize their structure and associated signaling mechanisms, it would be logical to expect the nucleus, as an adaptive and dynamic organelle, to participate in similar events including βcatenin, YAP and MKL1-SRF signaling. To this end, future studies should consider the nuclear envelope and LINC complexes as an integral part of the cellular mechanosensory mechanisms that regulate biochemical and physical coupling of the cell to its physical environment.
As both tissues, and the cells that comprise them, adapt to mechanical challenges, the type of mechanical input will dictate the adaptive cytoskeletal architecture and associated signaling responses. While there remain numerous challenges within the field, such as under-standing the intricate manner in which mechanical signals interact with hormones or pharmacological interventions, mechanical input has the potential to regulate cell and tissue functions in both healthy and diseased states. As such, these and future studies will provide insight into how mechanical force guides growth and repair of skeletal tissues. Many questions remain including why aged cells may be resistant to physical signals, how force can be integrated into tissue engineering applications, and which exercise regimens are provide optimal responses for specific disease states. In sum, it is critical that both scientists and clinicians understand the potential of mechanical forces to alter cellular and tissue response, with the ultimate goal of harnessing these signals for the repair and regeneration of injured or diseased tissues.
Significance Statement.
This concise review details the ability of stem cells to regulate their lineage allocation based on mechanical forces exerted upon them. The mechanical qualities of both the external environment (the extracellular matrix) and the internal cytoskeleton of stem cells alter how they respond. Additionally, forces are translated into biochemical signals using different molecular machinery at the plasma membrane and nuclear membrane. These distinctions have been outlined in this paper and the consequent diseases have been described
Acknowledgments
Funding support: This study was supported by NSBRI PF04304 through NASA NCC 9-58, AR066616, EB014351, AR056655, and AR064133.;
Footnotes
Conflict of Interest: All authors have no conflicts of interest.;
Author Contributions
G.U.: concept/design, manuscript writing and editing, final approval of manuscript; R.F.: concept/design, manuscript editing, final approval of manuscript; J.R.: concept/design, financial support, manuscript writing and editing, final approval of manuscript; W.T.: concept/design, manuscript writing and editing, final approval of manuscript
References
- 1.Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilak F, Cohen DM, Estes BT, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell stem cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen B, Guilluy C, Xie Z, et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem cells. 2011;29:1829–1836. doi: 10.1002/stem.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. Journal of cellular biochemistry. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Sen B, Xie Z, Case N, et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. Journal of biomechanics. 2011;44:593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyman S, Gilmore A, Burridge K, et al. Integrin-mediated activation of focal adhesion kinase is independent of focal adhesion formation or integrin activation. Studies with activated and inhibitory beta3 cytoplasmic domain mutants. The Journal of biological chemistry. 1997;272:22538–22547. doi: 10.1074/jbc.272.36.22538. [DOI] [PubMed] [Google Scholar]
- 8.Sen B, Xie Z, Case N, et al. mTORC2 Regulates Mechanically Induced Cytoskeletal Re-organization and Lineage Selection in Marrow-Derived Mesenchymal Stem Cells. Journal of Bone and Mineral Research. 2014;29:78–89. doi: 10.1002/jbmr.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi ML, Jaalouk DE, Shanahan CM, et al. The Interaction between Nesprins and Sun Proteins at the Nuclear Envelope Is Critical for Force Transmission between the Nucleus and Cytoskeleton. Journal of Biological Chemistry. 2011;286:26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113–1121. doi: 10.1016/j.cub.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010;11:567–578. doi: 10.1038/nrm2944. [DOI] [PubMed] [Google Scholar]
- 12.Cao K, Graziotto JJ, Blair CD, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Science translational medicine. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 13.Dawson JI, Kanczler J, Tare R, et al. Concise review: bridging the gap: bone regeneration using skeletal stem cell-based strategies - where are we now? Stem cells. 2014;32:35–44. doi: 10.1002/stem.1559. [DOI] [PubMed] [Google Scholar]
- 14.Burridge K, Wittchen ES. The tension mounts: stress fibers as force-generating mechanotransducers. The Journal of cell biology. 2013;200:9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessey EC, Guilluy C, Burridge K. From mechanical force to RhoA activation. Biochemistry. 2012;51:7420–7432. doi: 10.1021/bi300758e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang N, Butler JP, Ingber DE. Mechanotransduction Across the Cell-surface and Through the Cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 17.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 18.Pelham RJ, Wang Y-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBeath R, Pirone DM, Nelson CM, et al. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Chu JS, Tsou AD, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobaa S, Hoehnel S, Lutolf MP. Substrate elasticity modulates the responsiveness of mesenchymal stem cells to commitment cues. Integr Biol (Camb) 2015 doi: 10.1039/c4ib00176a. [DOI] [PubMed] [Google Scholar]
- 22.Engler AJ, Sen S, Sweeney HL, et al. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 23.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science. 2013:341. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong J-H, Hwang ES, McManus MT, et al. TAZ, a Transcriptional Modulator of Mesenchymal Stem Cell Differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 25.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Rowe RG, Botvinick EL, et al. MT1-MMP-dependent control of skeletal stem cell commitment via a beta1-integrin/YAP/TAZ signaling axis. Developmental cell. 2013;25:402–416. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobusue H, Onishi N, Shimizu T, et al. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat Commun. 2014:5. doi: 10.1038/ncomms4368. [DOI] [PubMed] [Google Scholar]
- 28.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science. 2013;340:864–867. doi: 10.1126/science.1235038. [DOI] [PubMed] [Google Scholar]
- 30.Plessner M, Melak M, Chinchilla P, et al. Nuclear F-actin formation and reorganization upon cell spreading. J Biol Chem. 2015;290:11209–11216. doi: 10.1074/jbc.M114.627166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen B, Xie Z, Uzer G, et al. Intranuclear Actin Regulates Osteogenesis. Stem Cells. 2015 doi: 10.1002/stem.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin J, Schwartz Z, Boyan BD, et al. Caveolin-1 Knockout Mice Have Increased Bone Size and Stiffness. Journal of Bone and Mineral Research. 2007;22:1408–1418. doi: 10.1359/jbmr.070601. [DOI] [PubMed] [Google Scholar]
- 33.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim TJ, Joo C, Seong J, et al. Distinct mechanisms regulating mechanical force-induced Ca(2)(+) signals at the plasma membrane and the ER in human MSCs. Elife. 2015;4:e04876. doi: 10.7554/eLife.04876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson WR, Majid AS, Czymmek KJ, et al. Association of the α2δ1 subunit with Cav3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. Journal of Bone and Mineral Research. 2011;26:2125–2139. doi: 10.1002/jbmr.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marjoram RJ, Lessey EC, Burridge K. Regulation of RhoA activity by adhesion molecules and mechanotransduction. Curr Mol Med. 2014;14:199–208. doi: 10.2174/1566524014666140128104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burridge K, Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell motility. 1983;3:405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- 38.Burridge K, Mangeat P. An interaction between vinculin and talin. Nature. 1984;308:744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- 39.Guilluy C, Swaminathan V, Garcia-Mata R, et al. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 41.Thompson WR, Guilluy C, Xie Z, et al. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem cells. 2013;31:2528–2537. doi: 10.1002/stem.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang P, Wu Y, Jiang Z, et al. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. International journal of molecular medicine. 2012;29:1083–1089. doi: 10.3892/ijmm.2012.934. [DOI] [PubMed] [Google Scholar]
- 43.Case N, Thomas J, Sen B, et al. Mechanical regulation of glycogen synthase kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein. The Journal of biological chemistry. 2011;286:39450–39456. doi: 10.1074/jbc.M111.265330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Case N, Xie Z, Sen B, et al. Mechanical activation of β-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. Journal of Orthopaedic Research. 2010;28:1531–1538. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 46.Castillo AB, Leucht P. Bone Homeostasis and Repair: Forced Into Shape. Current rheumatology reports. 2015;17:537. doi: 10.1007/s11926-015-0537-9. [DOI] [PubMed] [Google Scholar]
- 47.Lee KL, Guevarra MD, Nguyen AM, et al. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4:7. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tummala P, Arnsdorf EJ, Jacobs CR. The Role of Primary Cilia in Mesenchymal Stem Cell Differentiation: A Pivotal Switch in Guiding Lineage Commitment. Cellular and molecular bioengineering. 2010;3:207–212. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoey DA, Tormey S, Ramcharan S, et al. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem cells. 2012;30:2561–2570. doi: 10.1002/stem.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahl KN, Booth-Gauthier EA, Ladoux B. In the middle of it all: Mutual mechanical regulation between the nucleus and the cytoskeleton. Journal of biomechanics. 2010;43:2–8. doi: 10.1016/j.jbiomech.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Skepper JN, Yang F, et al. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. Journal of cell science. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 52.Autore F, Pfuhl M, Quan X, et al. Large-Scale Modelling of the Divergent Spectrin Repeats in Nesprins: Giant Modular Proteins. PloS one. 2013;8:e63633. doi: 10.1371/journal.pone.0063633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart-Hutchinson PJ, Hale CM, Wirtz D, et al. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Experimental cell research. 2008;314:1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lombardi ML, Jaalouk DE, Shanahan CM, et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. The Journal of biological chemistry. 2011;286:26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Current opinion in cell biology. 2011;23:47–54. doi: 10.1016/j.ceb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Luxton GWG, Gomes ER, Folker ES, et al. Linear Arrays of Nuclear Envelope Proteins Harness Retrograde Actin Flow for Nuclear Movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khatau SB, Kusuma S, Hanjaya-Putra D, et al. The Differential Formation of the LINC-Mediated Perinuclear Actin Cap in Pluripotent and Somatic Cells. PloS one. 2012;7:e36689. doi: 10.1371/journal.pone.0036689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Versaevel M, Braquenier J-B, Riaz M, et al. Super-resolution microscopy reveals LINC complex recruitment at nuclear indentation sites. Sci Rep. 2014:4. doi: 10.1038/srep07362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munter S, Enninga J, Vazquez-Martinez R, et al. Actin polymerisation at the cytoplasmic face of eukaryotic nuclei. BMC Cell Biol. 2006;7:23. doi: 10.1186/1471-2121-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reinhard M, Zumbrunn J, Jaquemar D, et al. An α-Actinin Binding Site of Zyxin Is Essential for Subcellular Zyxin Localization and α-Actinin Recruitment. Journal of Biological Chemistry. 1999;274:13410–13418. doi: 10.1074/jbc.274.19.13410. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman LM, Jensen CC, Chaturvedi A, et al. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Molecular Biology of the Cell. 2012;23:1846–1859. doi: 10.1091/mbc.E11-12-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fedorchak GR, Kaminski A, Lammerding J. Cellular mechanosensing: Getting to the nucleus of it all. Progress in Biophysics and Molecular Biology. 2014;115:76–92. doi: 10.1016/j.pbiomolbio.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uzer G, Thompson WR, Sen B, et al. Cell Mechanosensitivity to Extremely Low-Magnitude Signals Is Enabled by a LINCed Nucleus. Stem cells. 2015;33:2063–2076. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chambliss AB, Khatau SB, Erdenberger N, et al. The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci Rep. 2013:3. doi: 10.1038/srep01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banerjee I, Zhang J, Moore-Morris T, et al. Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response. PLoS Genet. 2014;10:e1004114. doi: 10.1371/journal.pgen.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horn HF, Brownstein Z, Lenz DR, et al. The LINC complex is essential for hearing. The Journal of Clinical Investigation. 2013;123:740–750. doi: 10.1172/JCI66911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Codelia Veronica A, Sun G, Irvine Kenneth D. Regulation of YAP by Mechanical Strain through Jnk and Hippo Signaling. Curr Biol. 2014;24:2012–2017. doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Driscoll Tristan P, Cosgrove Brian D, Heo S-J, et al. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophys J. 108:2783–2793. doi: 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumann S, Schneider M, Daugherty RL, et al. Nesprin-2 Interacts with α-Catenin and Regulates Wnt Signaling at the Nuclear Envelope. Journal of Biological Chemistry. 2010;285:34932–34938. doi: 10.1074/jbc.M110.119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crisp M, Liu Q, Roux K, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. The Journal of cell biology. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Constantinescu D, Gray HL, Sammak PJ, et al. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 72.Akter R, Rivas D, Geneau G, et al. Effect of Lamin A/C Knockdown on Osteoblast Differentiation and Function. Journal of Bone and Mineral Research. 2009;24:283–293. doi: 10.1359/jbmr.081010. [DOI] [PubMed] [Google Scholar]
- 73.Tong J, Li W, Vidal C, et al. Lamin A/C deficiency is associated with fat infiltration of muscle and bone. Mechanisms of Ageing and Development. 2011;132:552–559. doi: 10.1016/j.mad.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Li W, Yeo LS, Vidal C, et al. Decreased bone formation and osteopenia in lamin a/c-deficient mice. PloS one. 2011;6:e19313. doi: 10.1371/journal.pone.0019313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vidal C, Bermeo S, Fatkin D, et al. Role of the nuclear envelope in the pathogenesis of age-related bone loss and osteoporosis. Bonekey Rep. 2012;1:62. doi: 10.1038/bonekey.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 77.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bermeo S, Vidal C, Zhou H, et al. Lamin A/C Acts as an Essential Factor in Mesenchymal Stem Cell Differentiation Through the Regulation of the Dynamics of the Wnt/beta-Catenin Pathway. J Cell Biochem. 2015;116:2344–2353. doi: 10.1002/jcb.25185. [DOI] [PubMed] [Google Scholar]
- 79.Verstraeten VLRM, Ji JY, Cummings KS, et al. Increased mechanosensitivity and nuclear stiffness in Hutchinson–Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–393. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guilluy C, Osborne LD, Van Landeghem L, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim DH, Wirtz D. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials. 2015;48:161–172. doi: 10.1016/j.biomaterials.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ho CY, Jaalouk DE, Vartiainen MK, et al. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2004;2:E231. doi: 10.1371/journal.pbio.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sen B, Xie Z, Case N, et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44:593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka SM, Li J, Duncan RL, et al. Effects of broad frequency vibration on cultured osteoblasts. Journal of biomechanics. 2003;36:73–80. doi: 10.1016/s0021-9290(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 86.Hu SH, Chen JX, Butler JP, et al. Prestress mediates force propagation into the nucleus. Biochemical and Biophysical Research Communications. 2005;329:423–428. doi: 10.1016/j.bbrc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 87.Poh Y-C, Shevtsov SP, Chowdhury F, et al. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nature communications. 2012;3:866. doi: 10.1038/ncomms1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uzer G, Thompson WR, Sen B, et al. Cell Mechanosensitivity to Extremely Low-Magnitude Signals Is Enabled by a LINCed Nucleus. Stem Cells. 2015;33:2063–2076. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lattanzi G. Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells. Biochemical Society transactions. 2011;39:1698–1704. doi: 10.1042/BST20110657. [DOI] [PubMed] [Google Scholar]
- 90.Hernandez L, Roux KJ, Wong ESM, et al. Functional Coupling between the Extracellular Matrix and Nuclear Lamina by Wnt Signaling in Progeria. Developmental Cell. 2010;19:413–425. doi: 10.1016/j.devcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lammerding J, Schulze PC, Takahashi T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. The Journal of Clinical Investigation. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Case N, Thomas J, Xie Z, et al. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone. 2013;52:454–464. doi: 10.1016/j.bone.2012.08.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Case N, Xie Z, Sen B, et al. Mechanical activation of beta-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28:1531–1538. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sen B, Xie Z, Case N, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mislow JMK, Holaska JM, Kim MS, et al. Nesprin-1α self-associates and binds directly to emerin and lamin A in vitro. FEBS Letters. 2002;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- 96.Tilgner K, Wojciechowicz K, Jahoda C, et al. Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of β-catenin. Journal of cell science. 2009;122:401–413. doi: 10.1242/jcs.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koike M, Kose S, Furuta M, et al. β-Catenin Shows an Overlapping Sequence Requirement but Distinct Molecular Interactions for Its Bidirectional Passage through Nuclear Pores. Journal of Biological Chemistry. 2004;279:34038–34047. doi: 10.1074/jbc.M405821200. [DOI] [PubMed] [Google Scholar]
- 98.Sharma M, Jamieson C, Johnson M, et al. Specific Armadillo Repeat Sequences Facilitate β-Catenin Nuclear Transport in Live Cells via Direct Binding to Nucleoporins Nup62, Nup153, and RanBP2/Nup358. Journal of Biological Chemistry. 2012;287:819–831. doi: 10.1074/jbc.M111.299099. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Scaffidi P, Misteli T. Lamin A-Dependent Nuclear Defects in Human Aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Z-J, Wang W-P, Chen Y-C, et al. Dysregulated interactions between lamin A and SUN1 induce abnormalities in the nuclear envelope and endoplasmic reticulum in progeric laminopathies. Journal of cell science. 2014;127:1792–1804. doi: 10.1242/jcs.139683. [DOI] [PubMed] [Google Scholar]
- 101.McClintock D, Ratner D, Lokuge M, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PloS one. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duque G, Rivas D. Age-related changes in lamin A/C expression in the osteoarticular system: Laminopathies as a potential new aging mechanism. Mechanisms of Ageing and Development. 2006;127:378–383. doi: 10.1016/j.mad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Duque G, Li W, Yeo LS, et al. Attenuated anabolic response to exercise in lamin A/C haploinsufficient mice. Bone. 2011;49:412–418. doi: 10.1016/j.bone.2011.04.023. [DOI] [PubMed] [Google Scholar]