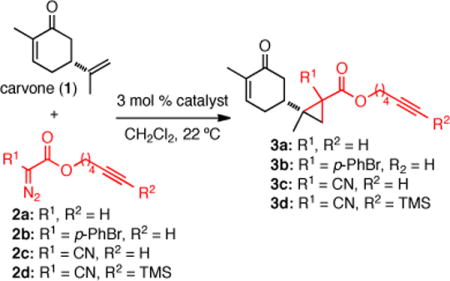
Table 1.
Screening of Catalysts and Diazo Reagents for the Cyclopropanation of Carvone (1).a
| ||||
|---|---|---|---|---|
|
| ||||
| entry | diazo | catalyst | % yieldb | drc |
| 1 | 2a | Cu(acac)2 | 0 | – |
| 2 | 2a | IprCuCl | trace | – |
| 3 | 2a | PdCl2(CH3CN)2 | 0 | – |
| 4 | 2a | Rh2(OCOCF3)4 | 20% | 2:1 |
| 5 | 2a | Rh2 (OAc)4 | 28% | 2:1 |
| 6 | 2a | Rh2 (esp)2 | 45% | 2:1 |
| 7 | 2b | Rh2 (esp)2 | 19% | 2:2:1:1 |
| 8 | 2c | Rh2(esp)2 | 55% | 1:1:1:1 |
| 9d | 2d | Rh2 (esp)2 | 91% | 1:1:1:1 |
Reaction of carvone (1) and diazo esters 2a–c, (4 equiv) at 22 °C.
Values refer to isolated yields.
Ratios were determined by 1H NMR (500 MHz); the relative stereochemistry was not determined.
Only 1.2 equiv of 2d were used. acac = acetylacetonate; IPr = [1,3-bis(diisopropylphenyl)imidazole-2-ylidene]; esp = α,α,α′,α′-tetramethyl-1,3-benzenedipropionate.
