Figure 1.
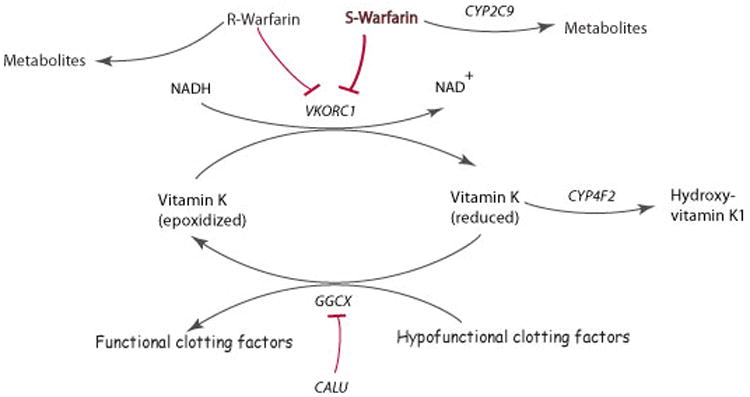
Schematic representation of warfarin metabolism and its mechanism of action.
Warfarin is administered via a racemic mixture of the R- and S- stereoisomers. S-warfarin is 3-5 times more potent than R-warfarin and is metabolized predominantly to 7- and 6- hydroxyl metabolites via CYP2C9. Warfarin exerts its anticoagulant effect through inhibition of its molecular target VKORC1, which in turn limits availability of reduced vitamin K, leading to decreased formation of functionally active clotting factors. These clotting factors are glycoproteins that are post-translationally carboxylated by gamma-glutamyl carboxylase (GGCX) to Gla-containing proteins. The endoplasmic reticulum chaperone protein calumenin (CALU) can bind to and inhibit GGCX activity. The metabolism of reduced vitamin K to hydroxyvitamin K1 is catalyzed by CYP4F2 which removes vitamin K from the vitamin K cycle (adapted from warfarin pharmacokinetics (PK) and pharmacodynamics (PD) pathways at PharmGKB, http://www.pharmgkb.org/do/serve?objId=PA451906&objCls=Drug#tabview=tab4).
