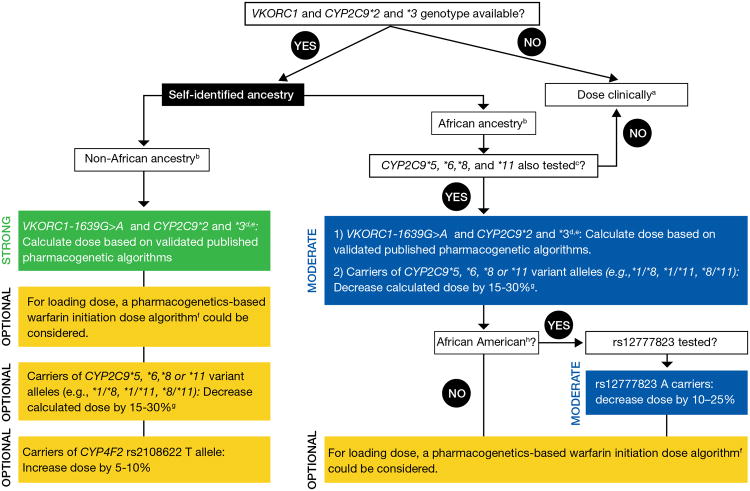
Figure 2. Dosing recommendations for Warfarin dosing based on genotype for adult patients.
a“Dose clinically” means to dose without genetic information, which may include use of a clinical dosing algorithm or standard dose approach
bData strongest for European and East Asian ancestry populations and consistent in other populations.
c45-50% of individuals with self-reported African ancestry carry CYP2C9*5,*6,*8,*11, or rs12777823. IF CYP2C9*5, *6, *8, and *11 WERE NOT TESTED, DOSE WARFARIN CLINICALLY. Note: these data derive primarily from African Americans, who are largely from West Africa. It is unknown if the same associations are present for those from other parts of Africa.
dMost algorithms are developed for the target INR 2-3.
eConsider an alternative agent in individuals with genotypes associated with CYP2C9 poor metabolism (e.g., CYP2C9*3/*3, *2/*3, *3/*3) or both increased sensitivity (VKORC1 A/G or A/A) and CYP2C9 poor metabolism.
fSee the EU-PACT trial for pharmacogenetics-based warfarin initiation (loading) dose algorithm (33) with the caveat that the loading dose PG algorithm has not been specifically tested or validated in populations of African ancestry.
gLarger dose reduction might be needed in variant homozygotes (i.e. 20-40%).
hAfrican American refers to individuals mainly originating from West Africa.