Figure 3. Regulation of the WNK-SPAK/OSR1-NCC pathway in the distal convoluted tubule.
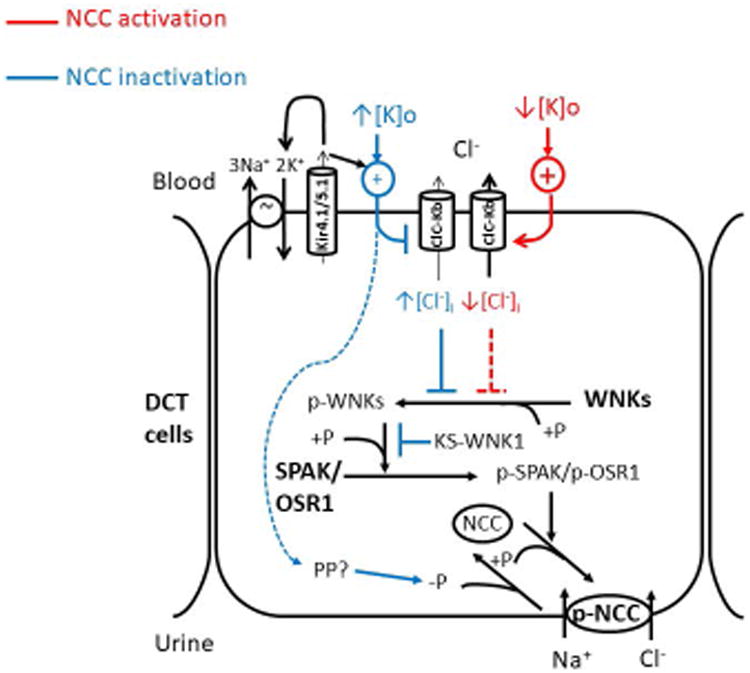
Autophosphorylation on the T-loop serine (S382 in WNK1, S335 in WNK4) activates WNK kinases. This process is inhibited by the Cl- ion binding to the Cl- sensing pocket. The plasma K+ level affects membrane potential (MP) and Cl- efflux via the ClC-Kb channel. Hypokalemia (↓[K]o) releases the Cl--sensing inhibition on WNK by hyperpolarizing MP, increasing Cl- efflux and reducing intracellular Cl- level ([Cl-]i). Hyperkalemia (↑[K]o) is supposed to do the opposite or inactivates NCC through an unknown SPAK/OSR1-independent protein phosphatase (PP) pathway (blue dashed arrow). Kir4.1/5.1 functions to maintain normal MP of distal convoluted tubule (DCT), together with the Na+, K+ ATPase and ClC-Kb channel. Inhibition of Kir4.1 results in a depolarized MP and increased intracellular Cl- concentration. Activated WNKs switch on SPAK/OSR1-NCC signaling through a phosphorylation cascade. Other kinases may phosphorylate NCC since Spak and Osr1 double knockout mice still preserved some phosphorylated NCC. KS-WNK1 may exert competitive inhibition on WNK1 through the interaction with WNK1 downstream substrates. Red lines and blue lines denote stimulatory and inhibitory regulations on NCC, respectively. +P: phosphorylation; -P: dephosphorylation.
