Abstract
The 5-HT6R has been considered as an attractive therapeutic target in the brain due to its exclusive expression in the brain. However, the mechanistic linkage between 5-HT6Rs and brain functions remains poorly understood. Here, we examined the effects of 5-HT6R-mediated cell morphological changes using immunocytochemistry, Western blot, and live-cell imaging assays. Our results showed that the activation of 5-HT6Rs caused morphological changes and increased cell surface area in HEK293 cells expressing 5-HT6Rs. Treatment with 5-HT specifically increased RhoA-GTP activity without affecting other Rho family proteins, such as Rac1 and Cdc42. Furthermore, live-cell imaging in hippocampal neurons revealed that activation of 5-HT6Rs using a selective agonist, ST1936, increased the density and size of dendritic protrusions along with the activation of RhoA-GTP activity and that both effects were blocked by pretreatment with a selective 5-HT6R antagonist, SB258585. Taken together, our results show that 5-HT6R plays an important role in the regulation of cell morphology via a RhoA-dependent pathway in mammalian cell lines and primary neurons.
Keywords: 5-HT6R, dendritic protrusions, live-cell imaging, morphology, serotonin, RhoA-GTP
INTRODUCTION
The neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) plays an important role in the regulation of numerous events in the central nervous system. 5-HT is related to physiological and pathophysiological events such as depression, anxiety, schizophrenia, and obesity (Filip and Bader, 2009) and mediates multiple physiological functions through seven subfamilies, 5-HT1–7 receptors (Yun and Rhim, 2011). The 5-HT6R is highly enriched in accumbens, cortex, hippocampus, and striatum which are involved in mood changes, cognition, learning and memory (Lorke et al., 2006; Woolley et al., 2004). To elucidate 5-HT6R-mediated signaling pathways, we serially found and characterized several proteins which interact with the intracellular domains of human 5-HT6Rs (Kim et al., 2014; Yun et al., 2007; 2010). First, we found that 5-HT6Rs activated the extracellular signal regulated kinase1/2 (ERK1/2) through a direct interaction with Fyn-dependent pathway, and 5-HT6R-Fyn protein complexes may play important roles in memory formation-related neuronal functions (Yun et al., 2007). As a novel function of 5-HT6Rs, we demonstrated that the 5-HT6R plays an important role in cell protection via interaction with Jun activation domain-binding protein-1 (Jab1) in the brain (Yun et al., 2010). Furthermore, our recent study suggested that the 5-HT6R is regulated by microtubule-associated protein 1B (MAP1B) and involved in the desensitization and trafficking that consequently control 5-HT6R-mediated signal transduction (Kim et al., 2014). Recently, Duhr et al. (2014) also identified several proteins interacting with the 5-HT6R, including Cdk5 and a number of its substrates known to regulate actin cytoskeleton dynamics.
As members of the same GS-protein-coupled receptors, the capability of 5-HT4 and 5-HT7 receptors to regulate cell morphology was previously reported in NIH3T3 cells and neuronal primary cultures (Kvachnina et al., 2005; Ponimaskin et al., 2007; Speranza et al., 2015). However, these data demonstrated distinct roles of G12-protein family (Gα12 and Gα13 subunits) for 5-HT4R and 5-HT7R-mediated cell morphological changes. It has been revealed that 5-HT7R is involved in the control of neuronal morphology via G12-protein-mediated stimulation of the small GTPase Cdc42 (Kvachnina et al., 2005; Ponimaskin et al., 2007). However, the precise role of 5-HT6Rs in controlling cell morphology and its signal pathways are not fully elucidated yet. To address these questions, we have here assessed 5-HT6R-mediated cellular change by Rho family GTPases that regulate numerous responses, such as change of actin-based cell morphology, and dendritic protrusion density in hippocampal neurons (Quilliam et al., 1995). Rho family GTPases act as key switches to regulate signal transduction pathways that link surface receptors to the actin cytoskeleton (Mouawad et al., 2013). In particular, RhoA controls actin organization, arrangement of stress fibers, change of cell morphology, and attachment (Jatho et al., 2015). In the present study, we showed that 5-HT6R-mediated RhoA-dependent cellular morphological changes occur in mammalian cell lines and primary cultured neurons.
MATERIALS AND METHODS
Antibodies and reagents
Anti RhoA, Cdc42, Rac1, and β-tubulin antibodies were purchased from Cell Signaling Technology (USA). ST1937 and SB258585 were obtained from Tocris (Bristol, UK). C3 transferase was from Cytoskeleton Inc. (USA). Y-27632 was a product of Invitrogen (USA). PP2, H89, and PD98059 were products of Calbiochem (USA). 5-HT and other chemicals were purchased from Sigma (USA). Protease inhibitor mixture were from BioVision (USA).
Cell culture and transfection
HEK293 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. HEK293 cells were transiently transfected with 5-HT6R-EGFP and 5-HT6R (ΔCT)-EGFP using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Briefly, HEK293 cells were seeded in 12-well culture plates in 1 ml of growth medium without antibiotics. For transfection, Lipofectamine 2000 was diluted in the DMEM medium without serum and incubated at room temperature (RT). The diluted Lipofectamine 2000 was then mixed with the diluted plasmid DNA (resulting concentration 0.75–1 μg), and the medium (transfection complex) was further incubated for 20 min at RT. Next, 200 μl of transfection complex was added to each well containing cells and the medium was incubated at 37°C in humidified 5% CO2 for 24 h. For neuronal cell culture, cultured hippocampal and cortical neurons were prepared from E18 Sprague-Dawley rat embryos and maintained for 12–17 days in vitro (DIV) (Cho et al., 2015; Kim et al., 2007). Neurons were transfected between 10–15 DIV using the Lipofectamine 2000 for 36–48 h for expression of 5-HT6R-EGFP or 5-HT6R-ΔCT-EGFP.
Immunocytochemistry
Fixed cells were washed three times with phosphate-buffered saline (PBS) and blocked in blocking solution (2% BSA, 0.1% Triton X-100, and 0.1% sodium azide in PBS). 5-HT6R-EGFP was labeled with mouse anti-GFP antibodies (Cell signaling, 1:500), followed by labeling with anti-mouse FITC (Abcam, 1:2000). All secondary antibodies were incubated at room temperature (RT) for 1 h. Cells were labeled with Alexa Fluor 555 Phalloidin (Molecular Probes, USA) for immunostaining F-actin and were mounted on a glass slide using Gold antifade reagent (Invitrogen). Mounted cells were viewed with a confocal FV 1000 laser scanning microscope (Olympus, Japan). A 20× objective was used to record whole cells. Images were cropped and/or enlarged to show localizations in sufficient detail.
Rho family GTPase and cofilin assays
For RhoA activity assay, an immobilized GST-fusion protein, Rhotekin (Millipore, Germany), was used as a probe. The pulled down active RhoA, which binds to the Rho-binding domain of Rhotekin, was detected using anti-RhoA antibody (1:1000). The Rac1 and Cdc42 activities were examined using EZ-Detect™ Rac1 activation Kit (Pierce, USA). Cells were lysed in 25 mM Tris-HCl, 150 mM NaCl, 5 mM MgCl2, 1% NP-40, 1 mM DTT, and 5% glycerol at 4°C. Active Rac1 was pulled down with GST-fusion protein containing p21-binding domain of p21-activated protein kinase 1 in the presence of immobilized Glutathione Disc. GTP-bound form was detected by western blots with anti-Rac1 or Cdc42 antibodies (1:1000). For p-cofilin assay, equal amounts of lysates from cortical neurons and HEK293 cells were prepared and immunoblotted with anti-cofilin (1:1000, Cell Signaling) and anti-phospho-cofilin (1:1000, Cell Signaling) antibodies.
Western blot analysis
Protein samples were separated using 12% SDS-PAGE gel, and then transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with 5% skim milk solution containing Tris-buffered saline and 0.1% tween 20 for 1 h at RT. After blocking, these membranes were incubated with primary antibodies at 4°C overnight. Membranes were washed three times, followed by incubation with horseradish peroxide-conjugated secondary antibodies (Jackson Immuno Research) for 1 h at RT. After three wash, the signal was detected using an ECL kit (iNtron Bio-technology, Korea). The intensity of the immunoblotting bands was measured using the AlphaEase program (version 5.1; Alpha Innotech, USA).
Live-cell imaging
Live neurons transfected with 5-HT6R-EGFP were transferred to the imaging chamber (Live Cell Instrument, Korea), filled with imaging solution (mM: 120 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 15 glucose, 15 HEPES, pH 7.35) or neurobasal media without phenol red (Gibco, Life Technologies) and imaged at 32°C. Temperature was controlled by heating plate base connected to the imaging chamber. Confocal images were acquired using the Revolution XD System (Andor Technology) equipped with Yokogawa CSU-X1 spinning disk confocal unit, 488 nm solid state laser, 561 nm solid state laser, and Andor 6-line laser combiner. Images were obtained using a 60× (NA 1.4) and a 14-bit iXON3 DU-885 EMCCD camera (Andor Technology) through the Metamorph software program (Molecular Device Inc.). For the SB258585 and ST1936 treatment experiments (Figs. 3G–3J), neurons were maintained at basal states for 6 minutes, then pre-incubated with SB258585 for 20 min, and then treated with ST1936 for 30 min.
Fig. 3. Activation of 5-HT6Rs increases the number and size of dendritic protrusions in cultured hippocampal neurons.
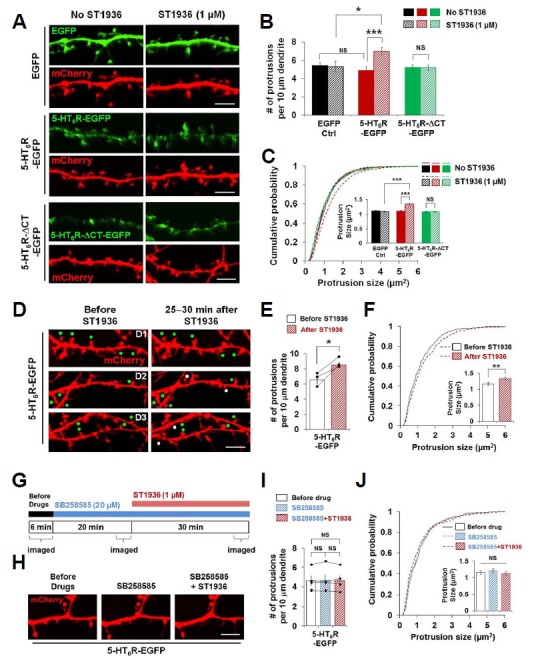
(A–F) Expression of 5-HT6R-EGFP, but not 5-HT6R-ΔCT-EGFP, exhibits ST1936-mediated morphological changes of dendritic protrusions. (A) Confocal images of neurons expressing EGFP, 5-HT6R-EGFP or 5-HT6R-ΔCT-EGFP together with mCherry. Neurons were transfected at DIV10–13 and imaged at DIV12–15. (B) Dendritic protrusion density was analyzed. Around 1,800 to 4,500 μm dendritic length for each was analyzed from 12, 12, 18, 16, 25, 23 neurons from left to right. (C) Cumulative distribution of individual dendritic protrusion size. Insets display means ± S.E. of individual dendritic protrusions. n = 1022, 746, 1391, 1554, 2239, 2038 protrusions from 12, 12, 18, 16, 25, 23 neurons from left to right. (D) Confocal live–cell images before and 25–30 minutes after ST1936 treatment. Three pairs of representative images (D1, D2, and D3) were shown. Neurons were co-transfected with 5-HT6R-EGFP and mCherry at DIV13 and live–cell imaged at DIV15. White and green dots indicate newly formed and growing dendritic protrusions, respectively, after ST1936 treatment. (E) Dendritic protrusion density was analyzed. Around 700 μm dendritic length was analyzed from 3 neurons. (F) Cumulative distribution of individual dendritic protrusion size. n = 446, 594 protrusions from 3 neurons. Student’s paired t-test was used for (F). (G–J) Blockade of 5-HT6Rs inhibits ST1936-mediated morphological changes of dendritic protrusions in cultured hippocampal neurons. (G) Experimental schematic diagram for effect of 5-HT6R inhibitor on ST1936-mediated morphological changes of dendritic protrusions. (H) Representative confocal images from live-cell imaging experiment from (G). (I) Dendritic protrusion density was analyzed. Around 600 μm dendritic length was analyzed from 4 neurons. (J) Cumulative distribution of individual dendritic protrusion size. n = 290, 291, 287 protrusions from 4 neurons. Student’s paired t test was used for (I). NS: not significant, *P < 0.05, **P < 0.01, *** P < 0.001. Scale bar, 5 μm.
Image analysis and quantification
For quantification of morphological changes of HEK293 cells, each image was equally scaled, and individual cell surface areas were measured using the Metamorph software program per single cell. For quantification of protrusion density, mCherry channels of each image were scaled equally for counting the number of total protrusions consisting of spines and filopodia. For the quantification of Figs. 3B and 3C, around 1,850 to 1,890 μm of dendritic length from 12 neurons each was analyzed for EGFP control samples. Between 2,300 and 3,000 μm of dendritic length from 16 to 18 neurons was analyzed for 5-HT6R-EGFP samples. Between 3,900 and 4,410 μm of dendritic length from 23 to 25 neurons was analyzed for 5-HT6R-ΔCT-EGFP samples. Around 700 μm of dendritic length from 3 neurons for Figs. 3E and 3F, and around 600 μm of dendritic length from 4 neurons for Figs. 3I and 3J were analyzed. For measurement of protrusion area, cell-filled mCherry images were equally scaled and protrusion areas including spine head and spine neck were measured.
Statistical analysis
Data was analyzed using the GraphPad Prism Version 6 program (GraphPad Software Inc., USA). All numeric values are represented as the mean ± SE. The statistical significance of the data was determined using a Student’s unpaired t-test unless otherwise indicated.
RESULTS AND DISCUSSION
5-HT induces changes in cell morphology
To investigate whether 5-HT6Rs induce changes in cell morphology, we examined the effect of 5-HT in HEK293 cells expressing 5-HT6R-EGFP by immunocytochemistry. As shown in Fig. 1A, in response to 5-HT, HEK293 cells changed cell shape in a time-dependent manner. Because actin cytoskeleton is critical for regulating cell morphology, cells were stained with Alexa Fluor555 Phalloidin, a high-affinity F-actin probe. We found that treatment with 1 μM 5-HT for 60 min dramatically increased cell size of HEK293 cells expressing 5-HT6R-EGFP, whereas cells transfected with EGFP only did not produce any morphological change (Supplementary Fig. S1A). However, pretreatment of cells with SB258585, a selective antagonist of 5-HT6Rs, for 15 min prevented the 5-HT-induced changes in morphology (Fig. 1B), suggesting that the change occurred in a 5-HT6Rdependent manner. To test whether SB258585 itself produces any effect on morphological changes, we performed experiments in HEK293 cells expressing 5-HT6Rs and found that there was no morphological change observed by the treatment with SB258585 (Supplementary Fig. S2). Furthermore, we examined whether the expression of 5-HT6R itself induces a morphological change due to previously reported its constitutive activity (Deraredj Nadim et al., 2016). As shown in Supplementary Figs. S1B–S1D, the expression of 5-HT6R itself did not produce morphological change as compared to cells transfected with EGFP alone.
Fig. 1. 5-HT stimulated cell morphology alteration in 5-HT6R-EGFP expressed HEK293 cells.
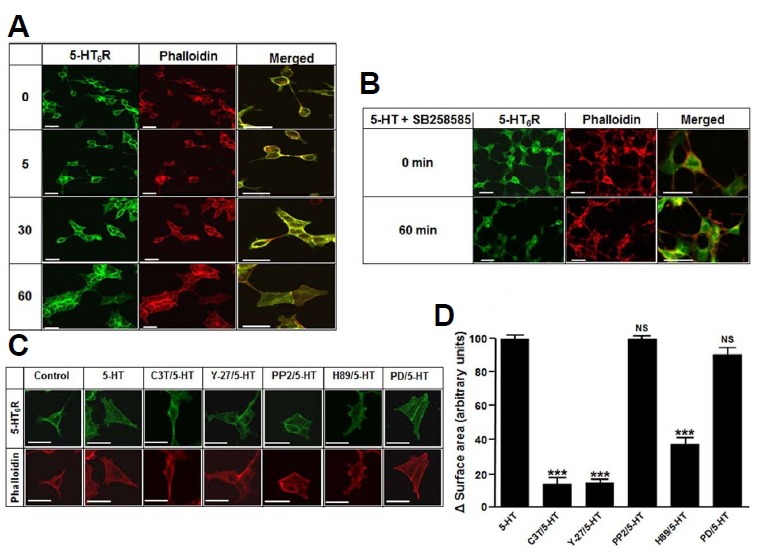
(A) HEK293 cells were transfected with 5-HT6R-EGFP. After 24 h transfection, cells were treated with 1 μM 5-HT for 0, 5, 30 and 60 min. Cells were fixed and immunostained with appropriated mouse anti-GFP antibodies, followed by labeling with anti-mouse FITC (green) or Alexa Fluor555 Phalloidin (red) for F-actin. (B) Cells were pretreated with 20 μM SB258585 for 15 min and then treated with 1 μM 5-HT for 60 min. Right panels of (A) and (B) show the enlarged merged images of the left and middle panels. (C) Cells were pretreated with C3 transferase (C3T, 1 μg/ml), Y-27632 (Y-27, 10 μM), PP2 (20 μM), H89 (20 μM), or PD98059 (PD, 10 μM) for 20 min. After pretreatment, cells were further incubated with 1 μM 5-HT for 60 min. (D) Quantification of morphological changes following each treatment. Cell areas were measured and presented as change of surface area per single cell. Values were normalized to the change of surface area in 5-HT-treated cells. NS: not significant, ***P < 0.001 vs. 5-HT-treated cells.
We examined several inhibitors of 5-HT6R-induced signal-transduction pathways demonstrated from our previous studies (Yun et al., 2007; 2010). The cells were pretreated with each inhibitors for 20 min, and the change of cellular morphology was observed after 60 min on treatment with 5-HT. As shown in Figs. 1C and 1D, pretreatment with the Rho inhibitor C3 transferase (C3T), the Rho kinase inhibitor Y-27632 (Y-27), or the PKA inhibitor H89 significantly attenuated 5-HT-mediated morphological changes. However, the Fyn inhibitor PP2 or the selective MEK inhibitor PD98059 (PD) did not affect 5-HT-mediated morphological changes. These results indicate that activation of 5-HT6Rs regulates cellular morphology via PKA- and Rho-dependent pathways in HEK293 cells.
The involvement of RhoA protein in 5-HT6R-mediated morphological changes
We next investigated whether the activation of 5-HT6Rs modulates Rho family GTPases which are key regulators of actin dynamics and neuronal morphology. We examined three the most well-known Rho family members, RhoA, Rac 1, and Cdc42 using western blot analysis and found that among them RhoA activities were strongly modulated by 5-HT. As shown in Fig. 2A, the level of RhoA-GTP, the active form of RhoA, was significantly and time-dependently increased upon the treatment with 5-HT in HEK293 cells expressing 5-HT6R-EGFP. RhoA-GTP level was started to increase from 5 min-treatment and maintained at significant level until 30 min. However, Rac1-GTP or Cdc42-GTP levels were not significantly changed upon the treatment with 5-HT under the indicated time (Figs. 2B and 2C). Therefore, these results suggest that the morphological alteration induced by 5-HT occurs through 5-HT6R-mediated RhoA pathways. We previously demonstrated that the C-terminal (CT, amino acids 321–440) region of 5-HT6Rs plays a pivotal role in 5-HT6R-mediated signal pathways via providing a common binding site with its novel binding proteins (Duhr et al., 2014; Kim et al., 2014; Yun et al., 2007; 2010). Therefore, we examined the involvement of the CT region in 5-HT6R-induced morphological changes by expressing the CT region truncated form of 5-HT6R (5-HT6R-ΔCT-EGFP) instead of 5-HT6R-EGFP in HEK293 cells. As shown in Fig. 2D, while the increase of RhoA-GTP levels following 5-HT treatment was confirmed in 5-HT6R-EGFP expressing cells, this was not observed in the cells expressing 5-HT6R-ΔCT-EGFP. On the other hand, there was no significant increase of Rac1-GTP or Cdc42-GTP levels by 5-HT in both 5-HT6R and 5-HT6R-ΔCT expressed cells. Rho family GTPase activities were assayed after 5-min treatment with 5-HT. Figure 2E showed that 5-HT-induced cellular morphological alterations were not clearly observed in 5-HT6R-ΔCT-EGFP expressed cells until 60 min-treatment compared to the immunofluorescence images observed in Fig. 1A.
Fig. 2. 5-HT significantly upregulated RhoA activities but not Cdc42 and Rac1 activities.
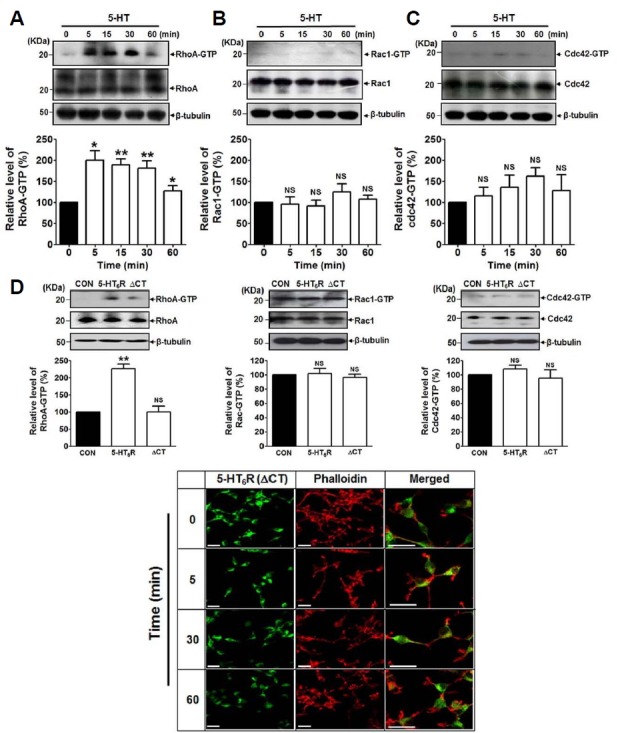
RhoA, Rac1 and Cdc42 activity assays were performed using GST-fusion protein, Rhotekin. Expression of RhoA-GTP, RhoA, Rac1-GTP, Rac1, Cdc42-GTP, Cdc42, and β-tubulin were determined by immunoblotting. Relative level of each Rho family GTPase band intensities was determined by Image J software, and each expression level was normalized to β-tubulin. (A-C) 5-HT6R-EGFP expressed HEK293 cells were treated with 1 μM 5-HT as indicated time points. Results are expressed as mean ± SE and representatives of three independent experiments (n = 3, NS: not significant. *P < 0.05, **P < 0.01). (D) Comparison of RhoA, Rac1, Cdc42 activities in non-transfected (CON), 5-HT6R-EGFP (5-HT6R)- or 5-HT6R-ΔCT-EGFP (ΔCT)-transfected cells. Rho family GTPase activities were assayed after 5-min treatment with 5-HT. Results are expressed as mean ± SE and representatives of three independent experiments (n = 3, NS: not significant. **P < 0.01). (E) Cells were grown and transiently transfected with 5-HT6R-ΔCT-EGFP (ΔCT) for 24 h. Immunofluorescence images were captured after 0, 5, 30, and 60 min treatment with 1 μM 5-HT.
Activation of 5-HT6R increases the number and size of dendritic protrusions in primary cultured neurons
We further tested whether the activation of 5-HT6Rs could modulate morphological changes in cultured neurons as we observed in HEK293 cells. In control hippocampal neurons expressing EGFP vector only, the treatment with ST1936, a selective agonist of 5-HT6Rs, did not cause any changes in morphological features such as dendritic protrusion density and size (Figs. 3A–3C), which might be due to scant amount of endogenous 5-HT6Rs in the cultured hippocampal neuronal system. However, no effect in the EGFP control condition implies that endogenous 5-HT6Rs may locate less in the spines, resulting in less signaling through the receptors by ST1936. Indeed, when we exogenously expressed 5-HT6R-EGFP in cultured hippocampal neurons, 5-HT6R activation by 1 μM ST1936 was sufficient enough to induce increase in dendritic protrusion density and size. In addition, consistent with the data shown in HEK293 cells (Fig. 2), 5-HT6R-ΔCTE-GFP expressed hippocampal neurons did not produce the ST1936-induced dendritic morphological changes, indicating that the CT region of 5-HT6Rs is required for ST1936-induced morphological changes of neuronal dendritic microstructures such as spines and filopodia. By performing livecell imaging techniques, we examined whether the neurons expressing 5-HT6R-EGFP exert the ST1936-induced morphological changes of dendritic protrusions in the presence of SB258585, an antagonist of 5-HT6Rs. In live neurons expressing 5-HT6R-EGFP, the treatment with ST1936 increased dendritic protrusion density and also enhanced protrusion size at the single protrusion level (Figs. 3D–3F). However, these increases were not observed under the pretreatment with SB258585 (Figs. 3G–3J).
The activation of 5-HT6Rs increase RhoA and cofilin activities in primary cultured neurons
To confirm the morphological effects of 5-HT6Rs transfected in neuronal cells, we finally examined whether the activation of neuronal 5-HT6Rs produces effects on RhoA activities. To perform RhoA activity assay as shown in Fig. 2, we carried immunoblot analysis using cultured cortical neurons for sufficient amount of sample proteins. Here, we showed that 5-HT6R activation by ST1936 significantly increased RhoA-GTP level, which was significantly blocked by the pretreatment with SB258585 in cultured neurons (Figs. 4A and 4B). As downstream targets of Rho proteins, RhoA proteins can also affect actin polymerization by regulating cofilin via Rho-ROCK-LIMK-cofilin pathway (Lin et al., 2003). Therefore, we further executed whether the activation of 5-HT6Rs modulates cofilin activities as a downstream target of RhoA. As shown in Figs. 4C and 4D, we observed that treatment with ST1936 significantly increased phosphorylation of cofilin, which was also significantly blocked by SB258585 in cultured neurons. We also confirmed that 5-HT increased cofilin activities in 5-HT6R-EGFP expressed HEK293 cells (Figs. 4E and 4F). In cultured neurons and HEK293 cells, treatment with SB258585 alone had no significant effect on RhoA or cofilin activation. Taken together, these results suggest that the 5-HT6R mediates cell morphology alterations via RhoA-cofilin-dependent pathways in the brain.
Fig. 4. 5-HT6Rs modulate neuronal RhoA and cofilin activities in agonist- and antagonist-dependent manners.
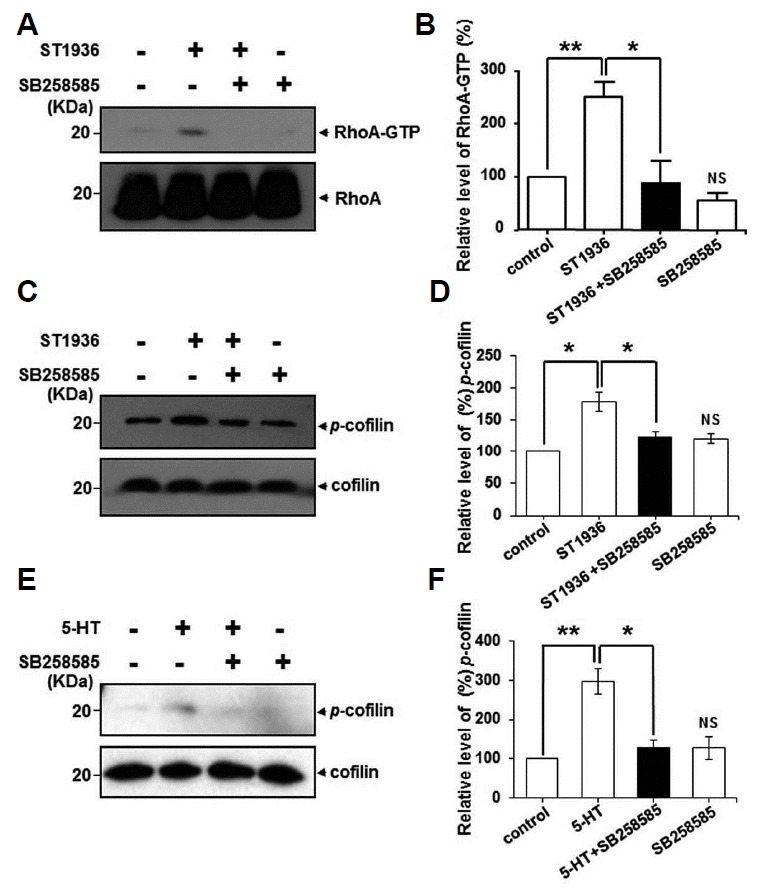
(A) 5-HT6R-EGFP transfected cortical neurons were treated with 1 μM ST1936 for 5 min in the absence or presence of 15 min pretreatment with 20 μM SB258585. Then RhoA activities were performed as describes in Fig. 2. The 5-HT-induced activation of p-cofilin was examined in 5-HT6R transfected cortical neurons (C) and 5-HT6R transfected HEK293 cells (E) after ST1965 or 5-HT treatment for 5 min, respectively. Activation of cofilin was determined as describes in materials and methods. (B, D, F) Relative level of each RhoA-GTP and p-cofilin band intensities was determined by Image J software, and each expression level was normalized to RhoA and cofilin, respectively. Results are expressed as mean ± SE and representatives of three independent experiments (n = 3, NS: not significant compare to control, *P < 0.05, **P < 0.01).
Based on the literature concerning the roles of Rho family GTPases in regulating actin dynamics and neuronal morphology, we, therefore, hypothesized that there is a functional linkage between 5-HT6Rs and Rho family GTPases and herein provided clear evidence for an involvement of RhoA-cofilin-dependent pathways in 5-HT6R-mediated morphological changes. In the present study, we found that activation of 5-HT6Rs caused morphological changes both in 5-HT6R-EGFP expressed HEK293 cells and cultured hippocampal neurons through the RhoA-GTP pathways. In addition, as far as our knowledge, the present work is the first report to show the effect of 5-HT6Rs on dendritic protrusion density and size in cultured hippocampal neurons using live cell imaging. Rho signaling has been implicated in the regulation of dendritic morphology, but the reported contributions of RhoA or its downstream RhoA kinase (ROCK) to these processes have varied greatly in different studies (Murakoshi et al., 2011; Nakayama et al., 2000). Therefore, for further experiments, it is necessary to test whether the effect of 5-HT6Rs on dendritic protrusions is prevented by RhoA/ROCK inhibitors in our culturing system. Although we surprisingly found scant amount of endogenous 5-HT6Rs in our primary neuronal culture systems, from high to moderate level, rat and human 5-HT6R mRNA and proteins were found in the cortex, hippocampus, nucleus accumbens, and striatum (Fone, 2008; Yun and Rhim, 2011). Therefore, in these brain areas, 5-HT6R agonist-mediated effects on dendritic protrusions may play roles for 5-HT6R signaling during neuronal structural and functional plasticity.
Furthermore, we provided an important involvement of the CT region of 5-HT6Rs in 5-HT6R-induced morphological changes. We previously found that this CT region plays an essential role in 5-HT6R-mediated signaling because it is a common binding region with 5-HT6R interacting proteins such as Fyn, Jab1 and MAP1B (Kim et al., 2014; Yun et al., 2007; 2010). Regarding the role of the CT regions in 5-HT6R-mediated morphological changes, the CT regions might serve as the key fragments to produce these effects by being involved in cytoskeleton transformation-related signal pathways or by providing a direct interaction with other proteins such as Fyn or MAP1B, which we already demonstrated, or other unknown binding proteins. However, the results from the PKA inhibitor H89-induced inhibition on 5-HT6R-mediated morphological changes (Fig. 1D) and no cAMP accumulation in cells transfected with 5-HT6R-ΔCT-EGFP (Supplementary Fig. S2) suggest that the increased cAMP level via the activation of 5-HT6Rs may be required in 5-HT6R-mediated morphological alteration. Therefore, rather than using the truncated receptor, it is necessary to examine other 5-HT6R mutants which have an intact C-terminal tail but a defect in cAMP activation. Interestingly, Jacobshagen et al. (2014) reported two types of mutants, 5-HT6R-Gsdead or 5-HT6R-D106A, in which 5-HT-induced cAMP signaling is abolished. Using these interesting mutants, it is essential to determine whether the C-terminal region of 5-HT6Rs is required for the morphological changes compared with the contribution of 5-HT-elicited cAMP signaling.
In conclusion, our results suggest that the 5-HT6R is involved in morphological changes and dendritic protrusion through RhoA-cofilin-dependent signal transduction pathway which might play an important role in mammalian neuronal functions in the brain.
Supplementary data
ACKNOWLEDGMENTS
This work was supported by the KIST Institutional Programs (Project No. 2E26820 and 2E26830) and the NRF Research Program (2016M3C7A1913845 and 2016H1D3A1908615). We thank Dr. Hongik Hwang and Melissa H. Park for proof-reading the manuscript.
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Cho E., Kim D.H., Hur Y.N., Whitcomb D.J., Regan P., Hong J.H., Park M. Cyclin Y inhibits plasticity-induced AMPA receptor exocytosis and LTP. Sci Rep. 2015;5:12624. doi: 10.1038/srep12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deraredj Nadim W., Chaumont-Dubel S., Madouri F., Cobret L., De Tauzia M.L., Zajdel P., Morisset-Lopez S. Physical interaction between neurofibromin and serotonin 5-HT6 receptor promotes receptor constitutive activity. Proc Natl Acad Sci USA. 2016;113:12310–12315. doi: 10.1073/pnas.1600914113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhr F., Deleris P., Raynaud F., Seveno M., Morisset-Lopez S., Mannoury la Cour C., Chaumont-Dubel S. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol. 2014;10:590–597. doi: 10.1038/nchembio.1547. [DOI] [PubMed] [Google Scholar]
- Filip M., Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep. 2009;61:761–777. doi: 10.1016/s1734-1140(09)70132-x. [DOI] [PubMed] [Google Scholar]
- Fone K.C.F. An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology. 2008;55:1015–1022. doi: 10.1016/j.neuropharm.2008.06.061. [DOI] [PubMed] [Google Scholar]
- Jacobshagen M., Niquille M., Chaumont-Dubel S., Marin P., Dayer A. The serotonin 6 receptor controls neuronal migration during corticogenesis via a ligand-independent Cdk5-dependent mechanism. Development. 2014;141:3370–3377. doi: 10.1242/dev.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatho A., Hartmann S., Kittana N., Mugge F., Wuertz C.M., Tiburcy M., Lutz S. RhoA ambivalently controls prominent myofibroblast characteritics by involving distinct signaling routes. PloS One. 2015;10:e0137519. doi: 10.1371/journal.pone.0137519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Yun H.M., Baik J.H., Chung K.C., Nah S.Y., Rhim H. Functional interaction of neuronal Cav1.3 L-type calcium channel with ryanodine receptor type 2 in the rat hippocampus. J Biol Chem. 2007;282:32877–32889. doi: 10.1074/jbc.M701418200. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Kim D.H., Lee K.H., Im S.K., Hur E.M., Chung K.C., Rhim H. Direct interaction and functional coupling between human 5-HT6 receptor and the light chain 1 subunit of the microtubule-associated protein 1B (MAP1B-LC1) PloS One. 2014;9:e91402. doi: 10.1371/journal.pone.0091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvachnina E., Liu G., Dityatev A., Renner U., Dumuis A., Richter D.W., Ponimaskin E.G. 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Zeng L., Liu Y., DeFea K., Schwartz M.A., Chien S., Shyy J.Y.J. Rho-ROCK-LIMK-cofilin pathway regulates shear stress activation of sterol regulatory element binding proteins. Circ Res. 2003;92:1296–1304. doi: 10.1161/01.RES.0000078780.65824.8B. [DOI] [PubMed] [Google Scholar]
- Lorke D.E., Lu G., Cho E., Yew D.T. Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 2006;7:36. doi: 10.1186/1471-2202-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouawad F., Tsui H., Takano T. Role of Rho-GTPases and their regulatory proteins in glomerular podocyte function. Can J Physiol Pharmacol. 2013;91:773–782. doi: 10.1139/cjpp-2013-0135. [DOI] [PubMed] [Google Scholar]
- Murakoshi H., Wang H., Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama A.Y., Harms M.B., Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponimaskin E., Voyno-Yasenetskaya T., Richter D.W., Schachner M., Dityatev A. Morphogenic signaling in neurons via neurotransmitter receptors and small GTPases. Mol Neurobiol. 2007;35:278–287. doi: 10.1007/s12035-007-0023-0. [DOI] [PubMed] [Google Scholar]
- Quilliam L.A., Khosravi-Far R., Huff S.Y., Der C.J. Guanine nucleotide exchange factors: activators of the Ras superfamily of proteins. Bioessays. 1995;17:395–404. doi: 10.1002/bies.950170507. [DOI] [PubMed] [Google Scholar]
- Speranza L., Giuliano T., Volpicelli F., De Stefano M.E., Lombardi L., Chambery A., Perrone-Capano C. Activation of 5-HT7 receptor stimulates neurite elongation through mTOR, Cdc42 and actin filaments dynamics. Front Behav Neurosci. 2015;9:62. doi: 10.3389/fnbeh.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley M.L., Marsden C.A., Fone K.C.F. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- Yun H.M., Rhim H. The serotonin-6 receptor as a novel therapeutic target. Exp Neurol. 2011;20:159–168. doi: 10.5607/en.2011.20.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H.M., Kim S., Kim H.-J., Kostenis E., Kim J.I, Seong J.Y., Rhim H. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem. 2007;282:5496–5505. doi: 10.1074/jbc.M606215200. [DOI] [PubMed] [Google Scholar]
- Yun H.M., Baik J.-H., Kang I., Jin C., Rhim H. Physical interaction of Jab1 with human serotonin 6 G-protein-coupled receptor and their possible roles in cell survival. J Biol Chem. 2010;285:10016–10029. doi: 10.1074/jbc.M109.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


