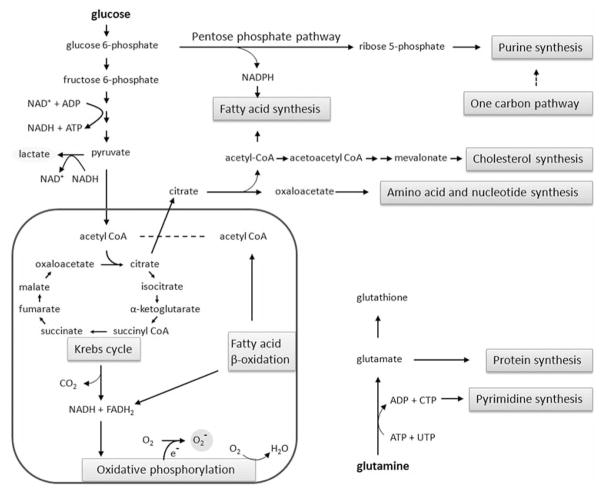
Figure 1.
The hypothesis that oxidative stress in pancreatic cancer cells is lower than that of normal duct cells. The formation of superoxide needs both oxygen and electron which is leaked from the oxidation of NADH and FADH2 by the respiratory chain in mitochondria. Enzymes in Krebs cycle and β-oxidation are down-regulated in pancreatic cancer cells in order to minimize the oxygen consumption, due to cells’ adaptation to hypoxia. As a result, less pyruvate will flux into mitochondria, and cancer cells adopt anaerobic lactate fermentation and elevated pentose phosphate pathway for biosynthesis of building blocks. Since the NADH generated from glycolysis will be largely consumed for the reduction of pyruvate and since less NADH and FADH2 will be generated by Krebs cycle and β-oxidation, less superoxide anion can be produced due to the availability of electron and oxygen in a hypoxic environment. Consequently, cancer cells express little manganese superoxide dismutase (SOD2) in mitochondria. Due to irreversible gene mutations in the cancer cells, the tumor-derived cell lines can maintain their metabolic phenotype in culture under regular conditions with ample oxygen, exhibiting aerobic glycolysis (Warburg effect).