Fig. 3.
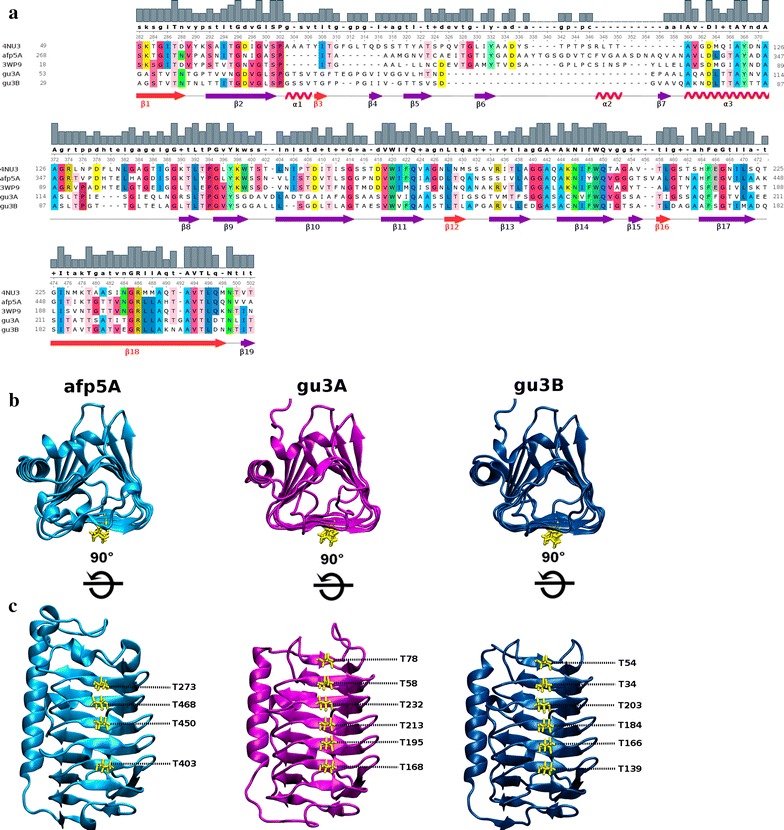
Cartoon representations of AFP models of afp5A (cyan), gu3A (magenta), and gu3B (blue). a Front view of triangular prisms. b Stereoviews showing the putative binding surface with ordered threonine residues (yellow). Images were generated using VMD. c Multiple sequence alignment of class III AFPs of the putative antifreeze proteins gu3A and gu3B (GU3.1.1) and afp5A (AFP5.1) and their respective templates for homology modeling, 3WP9 (hyperactive antifreeze protein from Colwellia sp.) and 4NU3, (hyperactive antifreeze protein from Flavobacterium frigoris). The region shown in the alignment corresponds only to the DUF3494 conserved domains of the sequences. Conserved amino acids in at least three of the aligned sequences are shown in colors, being each amino acid colored with a different color. Histogram bars and uppercase/lowercase letters in the consensus sequence indicate the level of conservation of the residues at each position of the alignment. Arrows and ribbons represent β-strands and α-helices, respectively. β-strands in red (β1, β3, β12, β16, β18) correspond to the β-strands located on the putative ice-binding surface of the AFPs
