Fig. 6.
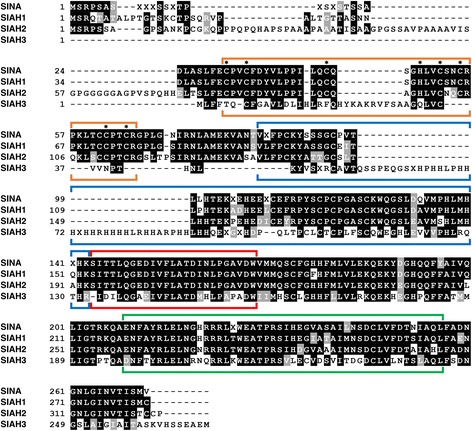
The consensus sequences of SINA, SIAH1, SIAH2, and SIAH3 were aligned to identify the invariant and divergent amino acid residues in this evolutionarily highly conserved SINA/SIAH E3 ligase family. There is a high level of amino acid conservation in the SBD domain in the SINA, SIAH1, SIAH2, and SIAH3 core consensus sequences in the SIAH family. The RING domain is marked by an orange bracket, the SZF domain by a blue bracket, the SBS by a red bracket, and the DIMER domain by a green bracket. Asterisks within the RING domain indicate the position of the invariant cysteine (Cys)/histidine (His) residues in SINA, SIAH1 and SIAH2. Amino acid positions marked with an “X” (instead of a valid one-letter amino acid abbreviation) indicate that the consensus at this site could not be resolved unambiguously. SINA, SIAH1, and SIAH2 share extensive sequence homology between each other in their core consensus sequences, whereas SIAH3 shows dramatic sequence divergence in the corresponding RING and SZF domains to those of SINA, SIAH1, and SIAH2 proteins. SINA, SIAH1, SIAH2, and SIAH3 share high levels of amino acid conservation in the SBS domains
