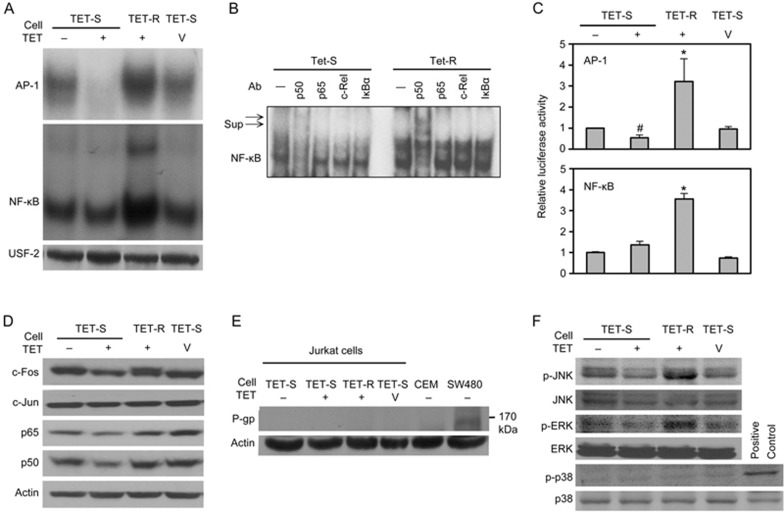
Figure 4.
MAPK/AP-1 and possible NF-κB activation, but not P-glycoprotein, contributes to TET resistance. TET-S and TET-R Jurkat cells (3×105 cells/mL) were seeded into 10-cm cell culture dishes and treated with 5 μmol/L TET or 0.1 mol/L HCL, as a vehicle control, for 24 h. (A) The DNA-binding activities of AP-1 and NF-κB in nuclear proteins were detected with an EMSA; USF-2 served as a loading control. (B) A supershift assay was used to determine the components in the NF-κB oligonucleotide-binding complex. The nuclear extracts were incubated with different mAbs for 30 min before incubation with radiolabelled NF-κB oligonucleotides. The mixtures were analysed with an EMSA. Sup indicates supershifts. c-Rel and IκBα were used as negative controls. (C) Cells (1×106 cells/mL) were transiently transfected with pNF-κB-luciferase or pAP-1-luciferase as well as pTK-Renilla-luciferase reporter plasmid. Eight hours after transfection, and 24 h after treatment with 5 μmol/L TET, cells were collected and analysed for luciferase activity in total lysates; untreated cells were used as a control. Activation of the reporter gene was expressed as fold-change relative to untreated Jurkat cells. *P<0.05 vs control. Cells (3×105 cells/mL) were incubated in 10-cm cell culture dishes in the presence of 5 μmol/L TET for 24 h; then, (D) p65, p50, c-Jun and c-Fos, and (F) total and phosphorylated JNK, ERK and p38 in equal amounts of total cell lysate protein (50 μg) for each sample were analysed by Western blotting. The results from a representative experiment are shown. Similar results were obtained in 2 other independent trials. (E) The expression of P-glycoprotein (P-gp) in cells with different levels of resistance to TET after treatment with or without TET for 24 h was evaluated by Western blotting. β-Actin was used as an internal control for equal loading. SW480 cells, which constitutively express P-gp, served as a positive control.