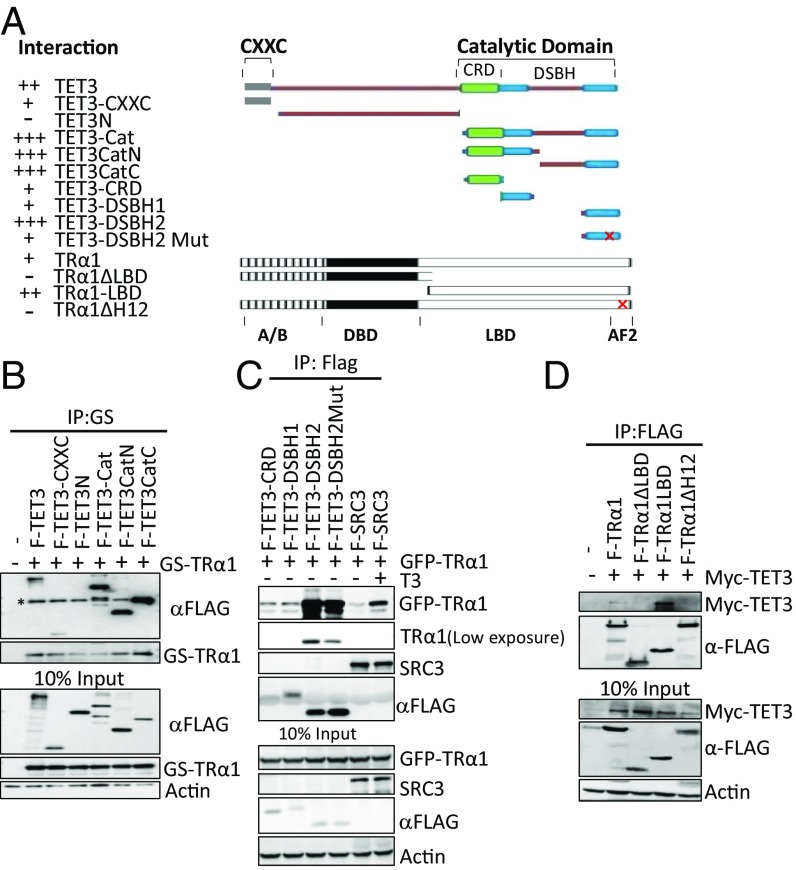
Fig. 2.
The catalytic domain of TET3 and the AF2 domain of TRα1 confer the interaction between these two proteins. (A) Schematic representation and summary of interactions of full-length and truncation mutants of TET3 and TRα1. AF2, activation function domain 2; Cat, catalytic domain; CRD, cysteine-rich domain; CXXC, CXXC domain; DSBH, double-stranded beta-helix domain; DBD, DNA binding domain; LBD, ligand binding domain. “+,” more “+,” and “−” represent, respectively, interaction, stronger interaction, and no interaction. (B and C) Characterization of the interacting regions in TET3 with TRα1. Whole-cell extracts were prepared from HEK293T cells expressing indicated proteins. (B) GS-TRα1 was precipitated with M280 beads; coprecipitated F-TET3 mutants were detected using anti-Flag antibody. Asterisk indicates bands for TRα1 that are recognized thanks to the cross-reaction between anti-Flag antibody and its GS tag. (C) Flag beads were used to precipitate TET3 truncation mutants; coprecipitated TRα1 was detected with anti-GFP antibody. (D) Characterization of the interacting regions in TRα1 with TET3. Flag beads were used to precipitate TRα1 truncation mutants; coprecipitated TET3 was detected with anti-Myc antibody.