Figure 5. The response of a microcircuit to co-transmission can be sculpted by feedback to the co-transmitting neuron.
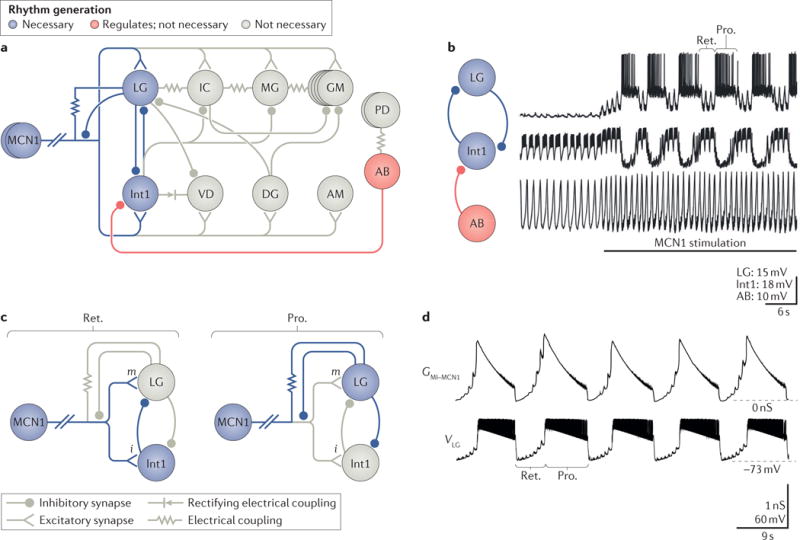
a| Modulatory commissural neuron 1 (MCN1) synaptic interactions with the gastric mill microcircuit are shown. The core rhythm generator includes the synaptic interactions between and intrinsic properties of MCN1, LG and Int1. These three neurons are necessary and sufficient to generate the gastric mill rhythm. The pyloric pacemaker interneuron AB regulates the gastric mill rhythm generator via its inhibitory synapse onto Int1. However, AB is not necessary for rhythm generation; the rhythm slows but continues when AB activity is eliminated119,134. The motor neurons shown in grey are not necessary for rhythm generation159, but some contribute to pattern generation via their intra-circuit synapses, and all contribute to movement via their synapses onto specific gastric mill muscles. The top row shows gastric mill protractor neurons (that is, motor neurons where their activation causes the teeth to move towards each other); the bottom row shows retractor neurons (that is, motor neurons, the activation of which causes the teeth to move away from one another and that are co-active with Int1 neuron); AB and PD are pyloric pacemaker neurons. All neurons are motor neurons except Int1 and AB, which are interneurons that project to the commissural ganglion (CoG). MCN1 excitation of Int1 is ionotropic; all other MCN1 co-transmitter actions are metabotropic. b | Tonic MCN1 stimulation drives the gastric mill rhythm (LG and Int1) and speeds up the pyloric rhythm (AB)118. MCN1 activation of the rhythm generator neurons LG and Int1 establishes the rhythmic alternating bursting pattern (LG bursting during the teeth protraction (Pro.) phase; Int1 bursting during the teeth retraction (Ret.) phase) that is then imposed on all gastric mill neurons. c | The gastric mill rhythm generator circuit during Ret. and Pro. phases of the MCN1-gastric mill rhythm118,127 is shown. During Ret. (left), MCN1 activity causes co-transmitter release, which drives Int1 activity, via ionotropic (i) excitation and provides a slow build-up of metabotropic (m) excitation that eventually enables LG to escape from Int1 inhibition and fire a burst of action potentials. The onset of a LG burst triggers the switch to the Pro. phase (right), during which LG is active and inhibits Int1. During Pro., LG activity also provides ionotropic inhibition to the stomatogastric ganglion (STG) terminals of MCN1 that prevents further co-transmitter release from MCN1 but enables MCN1 activity to sustain LG activity via an electrical synapse until the slowly decaying metabotropic excitation in LG falls below a critical level (see part d). In the figure, active neurons and synapses are shown in blue; silent neurons and synapses are shown in grey; the slanted double hashmarks indicate the abbreviated MCN1 axon. d | This part shows the output of a computational model showing the MCN1 Cancer borealis tachykinin-related peptide Ia (CabTRP Ia)-activated conductance GMI–MCN1 in the LG neuron waxing and waning during the gastric mill Ret. and Pro. phases, respectively (lower VLG trace)138. GMI–MCN1 increases during Ret. owing to continual CabTRP Ia release from MCN1, whereas it decays during Pro. owing to LG inhibition of the MCN1 terminals in the STG (see part c). The peak conductance occurs at the LG burst onset threshold, whereas the steep drop in conductance at the end of the LG burst occurs at the LG burst offset threshold. Part a is adapted with permission of Society for Neuroscience from: Convergent rhythm generation from divergent cellular mechanisms, Rodriguez J. C., Blitz D. M. & Nusbaum M. P., J. Neurosci. 33, 18047–18064, 2013; permission conveyed through Copyright Clearance Center, Inc. Part b is adapted with permission of Society for Neuroscience from: Coordination of fast and slow rhythmic neuronal circuits, Bartos M., Manor Y., Nadim F., Marder E. & Nusbaum M. P., J. Neurosci. 19, 6650–6660, 1999; permission conveyed through Copyright Clearance Center, Inc. Part c is adapted with permission from REF.134, Macmillan Publishers Limited. Part d is adapted with permission of Society for Neuroscience from: Parallel regulation of a modulator-activated current via distinct dynamics underlies comodulation of motor circuit output, DeLong N. D., Kirby M. S., Blitz D. M. & Nusbaum M. P., J. Neurosci. 29, 12355–12367, 2009; permission conveyed through Copyright Clearance Center, Inc.
