Figure 2.
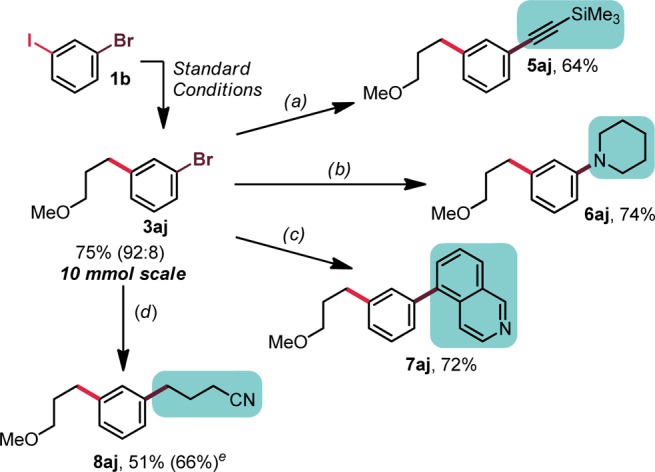
Diversification of 3aj. Conditions: (a) 3aj (0.5 mmol, 1 equiv), TMS-acetylene (3 equiv), Pd(PPh3)2Cl2 (5 mol %), CuI (5 mol %), Et3N (0.2 M), 80 °C, 48 h; (b) 3aj (1 mmol, 1 equiv), piperidine (1.5 equiv), XPhos G2 (2 mol %), Cs2CO3 (2.5 equiv), toluene/t-BuOH (5:1, 0.4 M), 80 °C, 18 h; (c) 3aj (0.5 mmol, 1 equiv), organotrifluoroborate (1.5 equiv), Pd(PPh3)2Cl2 (5 mol %), Cs2CO3 (3 equiv), THF/H2O (2:1, 0.25 M), 80 °C, 18 h; (d) 3aj (1 mmol, 1 equiv), alkylsilicate (1.2 equiv), [Ni(dtbbpy)(H2O)4]Cl2 (5 mol %), and Ru(bpy)3(PF6)2 (2 mol %), DMF (0.1 M), blue LEDs, rt, 24 h. (e) The yield in parentheses indicates the yield of sequential coupling, starting from 1b on 0.5 mmol scale without isolation of 3aj.
