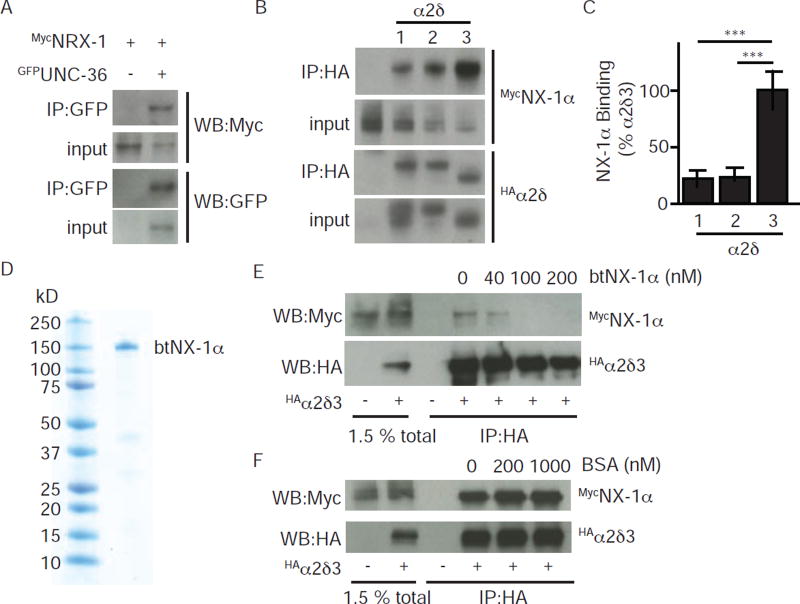
Figure 4. α-NX proteins bind with high affinity to α2δ proteins.
α-NX and α2δ proteins carrying the indicated epitope tags were expressed in transfected HEK cells and detected by immunoprecipitation and western blotting. Direct binding interactions were observed between α-NX and α2δ proteins from C. elegans (A) and rodents (B–F). Interaction with mouse NX-1α was observed for α2δ-1, 2, and 3 (B–C). Co-immunoprecipitation of rat NX-1α and α2δ-3 was efficiently blocked by adding purified recombinant cow Neurexin (btNX-1α) as a competitor (E). Co-immunoprecipitation was unaffected by adding an unrelated competitor (BSA, F). The ectodomain of btNX-1α was expressed as a soluble secreted protein in insect cells infected with a recombinant baculovirus. A coomassie stained gel of purified btNX-1α is shown (D). Representative gels (A,B,E,F) and quantitation of mouse NX-1α recovery in co-immunoprecipitates (C) are shown. Values that differ significantly are indicated (***, p <0.001; n.s., not significant). Error bars indicate SEM.