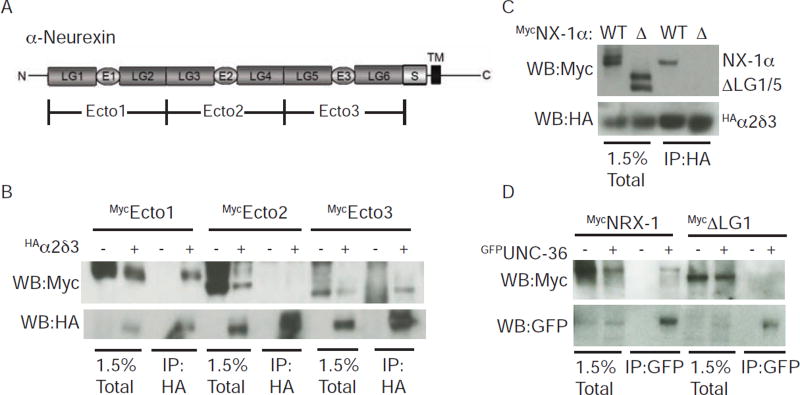
Figure 5. The LG1 and LG5 domains of α-NX mediate binding to α2δ proteins.
(A) A schematic is shown illustrating the domains found in α-NX proteins. Laminin-like globular domains (LG1-6), EGF-like domains (E1-3), the stalk region (S), and the transmembrane domain (TM) are indicated. This domain structure is found in mammalian NX-1α and in C. elegans NRX-1. Mouse NX-1α ectodomain fragments (Ecto1-3) used to map α2δ binding domains are indicated. (B–D) α-NX domains required for co-immunopreciptation with α2δ binding were mapped using mammalian (B–C) and C. elegans (D) proteins. For mouse NX-1α, a mutant lacking these domains (ΔLG1/5) failed to co-immunoprecipitate with α2δ-3 (C). A mutant NRX-1 lacking LG1 (ΔLG1) failed to co-immunoprecipitate with UNC-36/α2δ (D). Proteins carrying the indicated epitope tags were expressed in transfected HEK cells and detected by immunoprecipitation and western blotting. Further mapping of the α-NX binding domains are shown in Fig. S3.