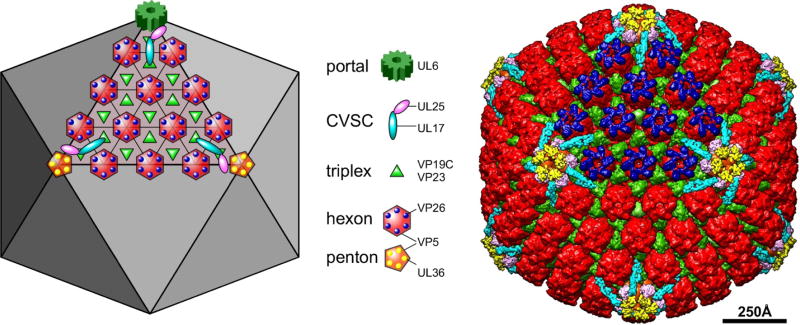
Figure 2. The capsid structural proteins.
The schematic diagram at left and the false-colored cryoEM reconstruction at right depict the locations of major and minor capsid components on one facet of the T=16 icosahedral lattice, as indicated by the legend. The portal complex, a dodecamer of the UL6 protein, occupies a unique penton vertex and functions both to nucleate capsid assembly as well as to provide the entry and exit of the dsDNA genome. It is not seen in cryoEM reconstructions where full icosahedral symmetry is imposed. Five copies of the CVSC molecule are situated around each vertex. The triplex molecule is a heterotrimer comprising one copy of VP19C and two copies of VP23. Hexamers of the major capsid protein, VP5, are capped by the small 12kDa VP26 subunit, while the pentamers bind a tegument protein, UL36, which also interacts with the UL25 subunit of the CVSC.