Abstract
Background
Cervical degenerative changes are the most common cause of cervical spondylotic myelopathy (CSM) and lower cervical instability (LCI). The purpose of this study was to investigate the associated factors of MRI signal changes and prognosis in single segmental CSM accompanied by LCI.
Material/Methods
A total of 122 patients met the inclusion criteria and were enrolled in this study with a minimum follow-up period of 2 years. According to the absence/presence of LCI, patients were divided into the unstable group (n=43 [35.2%]) and the stable group (n=79 [64.8%]). Clinical data and radiological parameters were compared between groups.
Results
The occurrence rate of increased signal intensity (ISI) of the spinal cord was 72.1% in the unstable group and 44.3% in the stable group, and the difference was significant. There were significant differences in preoperative JOA score, duration of symptoms, and number of physical signs between the 2 groups (p<0.001, =0.001 and <0.001, respectively). The recovery rate of the JOA score in the unstable group was significantly lower than in the stable group (p<0.001). Long duration of symptoms, low preoperative JOA score, and more preoperative physical signs were significantly correlated with low JOA recovery rate.
Conclusions
Patients suffering from CSM with LCI have higher incidence of ISI of the spinal cord. Longer duration of symptoms, lower preoperative JOA score, and more preoperative physical signs were highly predictive of poor surgical outcomes for patients with single segmental CSM with LCI.
MeSH Keywords: Intervertebral Disc Degeneration, Magnetic Resonance Imaging, Prognosis, Spine, Spondylosis
Background
Cervical spondylotic myelopathy (CSM) is a progressive degenerative disease of the cervical spine. The degenerative changes mostly include intervertebral disc degeneration and/or herniation, hyperosteogeny of cervical facet joints and rear edge of vertebral bodies, ossification of the longitudinal ligament (OPLL), and hypertrophy of the ligamentum flavum [1,2]. As the degeneration progress, the spinal cord is compressed and stimulated repeatedly, and the spinal cord blood supply is affected negatively. CSM is the most common cause of spinal cord dysfunction in older people [3,4], and often develops to irreversible nerve damage if not given timely treatment. Cervical instability refers to the excessive or abnormal activity of the cervical motion segment under physiological load, including rotational displacement and translational displacement [5]. Some previous studies demonstrated that patients with CSM often also have cervical instability [6,7].
Lower cervical instability (LCI) refers to the instability of cervical vertebrae section below C2–3. Dai [8] considered that cervical instability was a manifestation of the degenerative process of the cervical spine. Some previous studies reported that cervical instability may play an important role in the pathogenesis of CSM [7–9].
There have been many reports on CSM and segmental cervical instability [10,11] but few studies have focused on CSM accompanied by LCI. Anterior decompression surgery is generally considered to be an effective treatment for single segmental CSM, but the treatment for LCI is controversial. For the CSM patient with LCI for whom the unstable segment is not the pathogenic segment, does the unstable segment need to be treated at the same time during the operation? Is the incidence of ISI of spinal cord on MRI T2WI affected by LCI in CSM patients? To the best of our knowledge, there have been few reports addressing these questions.
The purposes of this research were to investigate: 1) the influence of LCI on incidence of ISI of the spinal cord on MRI T2WI in CSM patients; 2) the related factors affecting the surgical prognosis of anterior cervical decompression and fusion; and 3) the treatment strategy of single segmental CSM with LCI.
Material and Methods
Patients
Between January 2010 and June 2013, a total of 633 patients with CSM underwent operations at our hospital, but only 122 patients were eligible for final analysis in this study. The study consisted of 57 males and 65 females with a mean age of 62.3 years (range, 42–68 years). The mean follow-up period was 34.7 months (range, 24–53 months). The inclusion criteria were: (1) there are typical clinical symptoms and signs of CSM (e.g., motor and sensory dysfunction, positive Babinski sign and Hoffmann sign, tendon hyperreflexia); (2) the MRI showed obvious compression of the spinal cord or disc degeneration, and as a single segment; (3) the segment of ISI of spinal cord and intervertebral disc herniation was the same section; (4) all patients underwent ACDF, and the unstable segment was not processed in the unstable group. The exclusion criteria were: (1) previous history of spinal cord injuries or surgery of the cervical spine, cerebral infarction, cerebral blood blot, history of inflammation of the spinal cord, or peripheral nerve lesions; (2) cervical ossification of the posterior longitudinal ligament (OPLL); and (3) cervical disc herniation and cervical instability in the same section.
The research protocol was approved by the Research and Ethics Committee of The Third Hospital of Hebei Medical University, and all patients gave written informed consent for their information to be stored in the hospital database and used for this research.
Surgical procedure
All operations were performed by a single surgeon (Dr. Shen). Surgical procedures were carried out using the anterior approach via a right-side skin incision. For the purpose of adequate neural decompression, the posterior longitudinal ligament must be excised completely. Gentle decortication of the endplates was performed with a curette or burr. A suitable PEEK cage, filled with autologous bone fragments harvested from angularly excising adjacent vertebral bodies, was inserted into the disc space, and the anterior plate system was applied.
Clinical and radiological evaluations
Clinical data were prospectively collected preoperatively, postoperatively, and at final follow-up. The Japanese Orthopedic Association (JOA) scoring system was used to determine neurological functional status. The recovery rate was calculated by using the Hirabayashi method: The recovery rate (%)=(postoperative JOA score–preoperative score)/(17–preoperative score) ×100% (Table 1).
Table 1.
Evaluation of cervical myelopathy using a scoring system proposed by Japanese Orthopedic Association (JOA score) and recovery rate of JOA score.
| JOA score |
| I. Motor function of the upper extremity |
| 0. Impossible to eat with chopsticks or spoon |
| 1. Possible to eat with spoon, but not with chopsticks |
| 2. Possible to eat with chopsticks, but inadequate |
| 3. Possible to eat with chopsticks, but awkward |
| 4. Normal |
| II. Motor function of the lower extremity |
| 0. Impossible to walk |
| 1. Needs cane or aid on flat ground |
| 2. Needs cane or aid only on stairs |
| 3. Possible to walk without cane or aid, but slowly |
| 4. Normal |
| III. Sensory function |
| A. Upper extremity |
| 0. Apparent sensory loss |
| 1. Minimal sensory loss |
| 2. Normal |
| B. Lower extremity (same as A) |
| C. Trunk (same as A) |
| IV. Bladder function |
| 0. Complete retention |
| 1. Severe disturbance (sense of retention, dribbling, and incomplete continence) |
| 2. Mild disturbance (urinary frequency and urinary hesitancy) |
| 3. Normal |
Recovery rate of JOA score (Hirabayashi method).
Recovery rate (%)=(postoperative score–preoperative score)/(normal score (17)–preoperative score)×100
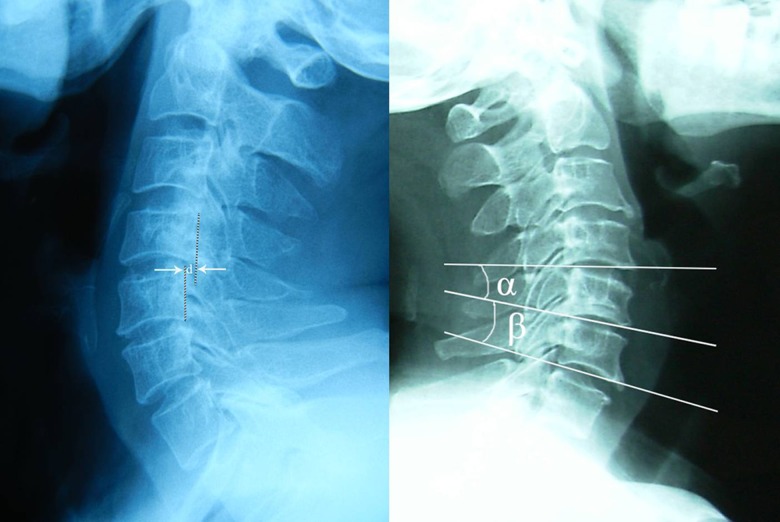
All patients underwent static and dynamic flexion/extension lateral radiographs preoperatively, postoperatively, and at final follow-up, which were used to assess LCI. Preoperative cervical MRI was performed to determine the spinal cord ISI and the spinal cord compression segment. All imaging data were independently analyzed by an orthopedic surgeon and an experienced radiologist through double-blind method. LCI was defined as: 1) Translational Instability: more than 3.5 mm horizontal displacement of 1 vertebra in relation to an adjacent vertebra measured on lateral roentgenograms; and 2) Rotational instability: more than 11 degrees of rotational difference to that of either adjacent vertebra measured on lateral roentgenograms [12] (Figure 1). According to the Yukawa’s grading criteria [13], ISI of spinal cord was classified into 3 groups based on sagittal T2-weighted images as follows: Grade 0, none; Grade 1, light (obscure); and Grade 2, intense (bright).
Figure 1.
Translational instability is defined as: more than 3.5 mm horizontal displacement of one vertebra in relation to an adjacent vertebra measured on lateral roentgenograms (d ≥3.5 mm, left). Rotational instability is defined as: more than 11 degrees rotational difference from that of either adjacent vertebra (α–β ≥11°, right).
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (version 22.0, SPSS Inc., Chicago, IL, USA). Results are presented as mean ±SD. All measurement data were evaluated using homogeneity of variance and normality tests. The differences between groups for age, follow-up period, JOA score, JOA recovery rate, duration of symptoms, and number of physical signs were analyzed using the Mann-Whitney U nonparametric test because they did not meet the criteria for normal distribution. Sex distribution and the incidence of ISI of the spinal cord between the 2 groups were compared using the chi-square analysis test, and multivariate stepwise logistic regression was used to analyze the risk factors related to JOA recovery rate. A p value <0.05 was considered statistically significant. A Kappa value was calculated for assessing the reliability of diagnosis as follows: 0–0.2, slight agreement; 0.21–0.4, fair agreement; 0.41–0.6, moderate agreement; 0.61–0.8, substantial agreement; and 0.81–1, excellent agreement.
Result
According to the presence/absence of LCI, the patients were divided into 2 groups: the unstable group (n=43, 23 females and 20 males) and the stable group (n=79, 42 females and 37 males).
Mean age was 51.2 years (range, 43–68 y) in the unstable group and 53.5 years (range, 42–65 y) in the stable group. Mean period of follow-up was 35.8 months (range, 24–53 m) in the unstable group and 33.9 months (range, 24–49 m) in the stable group. There were no significant differences in age, sex, and follow-up period between the 2 groups (P=0.457, 0.743, and 0.403, respectively). The preoperative, postoperative, and final follow-up JOA scores showed significant differences between the 2 groups (p<0.001, =0.003 and 0.011, respectively). A significantly difference was shown in duration of symptoms between the 2 groups (p=0,001). The recovery rate of JOA score at final follow-up for the unstable group was significantly less than that for the stable group (p<0.001). The ISI of spinal cord was evident in 31 of the 43 (72.1%) in the unstable group, and there were 12 patients in grade 0, 15 in grade 1, and 16 in grade 2. However, in the stable group, the increased signal intensity of the spinal cord existed in 35 of 79 (44.3%), with 44 in grade 0, 19 in grade 1, and 16 in grade 2. The occurrence rate of ISI of the spinal cord in the unstable group was significantly higher than in the stable group (p<0.001) (Table 2).
Table 2.
Clinical features and surgical outcomes in each group.
| Unstable group (n=43) | Stable group (n=79) | p-Value | |
|---|---|---|---|
| Age (years) | 51.2±8.51 | 53.5±9.24 | 0.457 |
| Sex(male: female) | 20/23 | 37/42 | 0.734 |
| Follow-up period (months) | 35.8±5.5 | 33.9±6.3 | 0.403 |
| The occurrence rate of ISI (%) | 72.1 (31/43) | 44.3 (35/79) | <0.001 |
| Duration of symptoms (months) | 25.4±11.1 | 18.2±7.8 | 0.001 |
| Number of physical signs | 2.89±0.73 | 2.24±0.75 | <0.001 |
| The JOA score (point) | |||
| Preoperative | 9.7±2.1 | 11.2±2.4 | <0.001 |
| Postoperative | 12.1±3.3 | 14.1±2.3 | 0.003 |
| Follow-up | 13.2±1.1 | 14.6±1.6 | 0.011 |
| Recovery rate of JOA score at the final follow-up (%) | 54.3±12.1 | 64.9±17.3 | <0.001 |
In the unstable group, motor dysfunction, sensory dysfunction, positive Hoffmann sign, positive Babinski sign, and tendon hyperreflexia was found in 33 cases, 38 cases, 35 cases, 28 cases, and 36 cases, respectively, as opposed to 42, 53, 49, 35, 51, respectively, in the stable group (Table 3). The mean number of physical signs was 2.89±0.73 in the unstable group and 2.24±0.75 in the stable group. There was a significant difference in the number of physical signs between the 2 groups (p<0.001).
Table 3.
The number of patients with typical CSM physical signs in each group.
| Physical sign | Unstable group | Stable group |
|---|---|---|
| Motor dysfunction | 33 | 42 |
| Sensory dysfunction | 38 | 53 |
| Positive Hoffmann sign | 35 | 49 |
| Positive Babiniski sign | 28 | 35 |
| Tendon hyperreflexia | 36 | 51 |
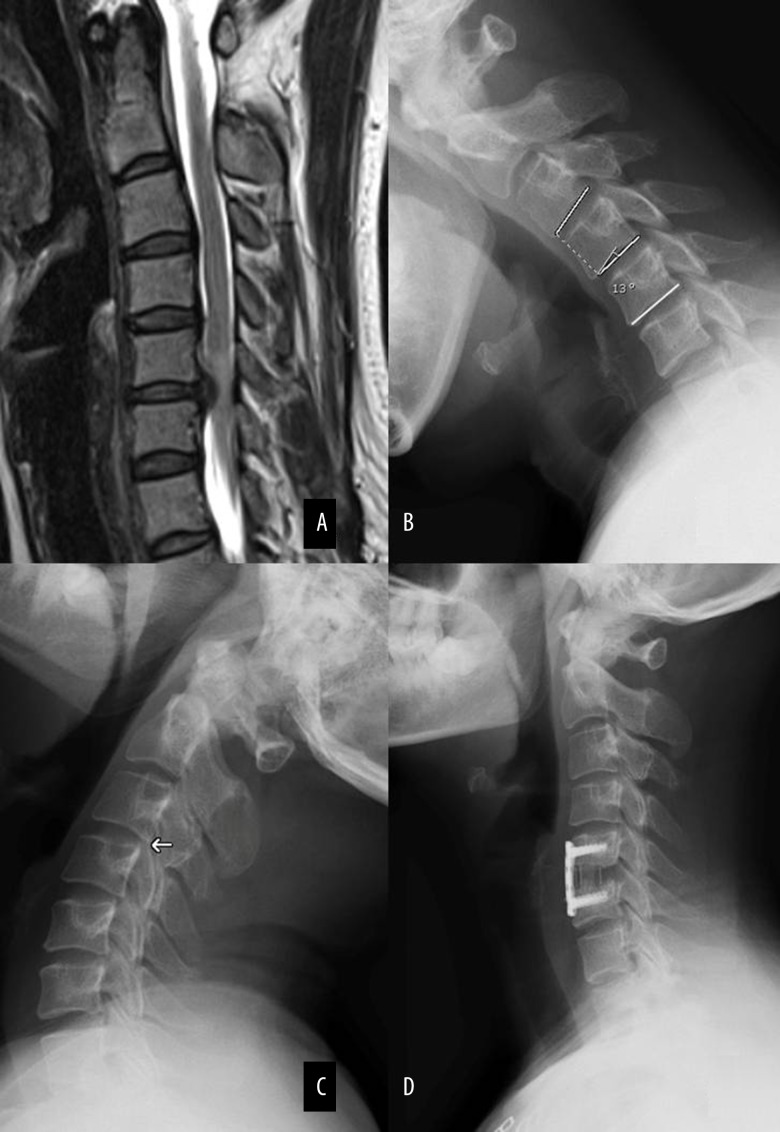
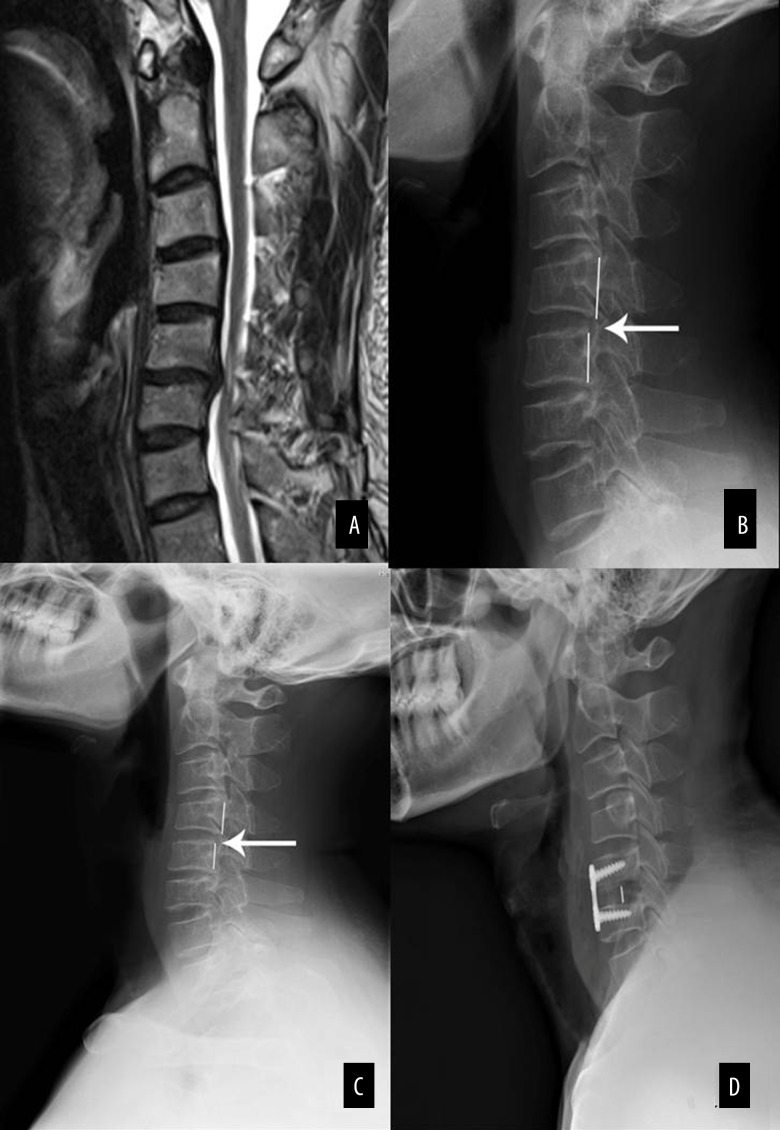
Figure 2 shows a representative 43-year-old female single segmental CSM patient with both translational instability and rotational instability on non-adjacent segments, who received single ACDF surgery treatment. Figure 3 shows a representative 52-year-old male single segmental CSM patient with translational instability on adjacent segment who received single ACDF surgery treatment.
Figure 2.
A 43-year-old female single segmental CSM patient with low cervical instability. Magnetic resonance image showing obvious spinal cord compression and ISI of the spinal cord (Grade 1) at C5–6 (A); Lateral radiograph showing C3–C4 instability with 13-degree rotational difference in flexion position (B) and 3.9 mm horizontal displacement in extension position (C); Postoperative radiograph showing single-level anterior cervical decompression and fusion at C5–6, while the unstable segment (C3–4) was not processed (D).
Figure 3.
A 52-year-old male single segmental CSM patient with low cervical instability. Magnetic resonance image showing obvious spinal cord compression and ISI of the spinal cord (Grade 1) at C5–6 (A); Lateral radiograph showing C4–C5 instability with 3.5 mm horizontal displacement in resting position (B) and 4.3 mm in extension position (C); Postoperative radiograph showing single-level anterior cervical decompression and fusion at C5–6, while the unstable segment (C4–5) was not processed (D).
There was no case of major neurological, vascular, or wound complications. In addition, there was no leakage of cerebrospinal fluid or persistent dysphagia in the 2 groups.
Multivariate stepwise logistic regression analysis showed that the best combination of surgical outcome predictors included preoperative JOA score, duration of symptoms, and number of preoperative physical signs. The result of multivariate stepwise logistic regression showed that longer duration of symptoms, lower preoperative JOA score, and more preoperative physical signs were significant risk factors for lower JOA recovery rate (Table 4).
Table 4.
Multivariate stepwise logistic regression for lower recovery ratio of JOA score.
| Measure | Odds ratio | 95% confidence intervals | P value |
|---|---|---|---|
| Preoperative JOA score | 2.369 | 1.328–3.764 | <0.001 |
| Duration of symptoms | 1.158 | 1.014–1.273 | <0.001 |
| Number of physical signs | 3.477 | 2.358–5.235 | 0.004 |
Assessment of the reliability of the diagnosis of cervical instability was done by an orthopedic surgeon and a radiologist. The Kappa value for cervical instability was 0.832, showing excellent agreement.
Discussion
ISI of spinal cord and prognosis of CSM with LCI
Although there have been numerous reports on ISI of the spinal cord, the pathogenesis is still unclear. ISI of the spinal cord on T2-weighted MRI is often seen in patients with cervical compressive myelopathy, which can reflect pathological changes in the spinal gray matter. Mizuno et al. [14] considered that cystic necrosis produced ISI of the spinal cord, caused by mechanical compression and venous infarction. Yagi et al. [15] found that ventral compression and cervical instability were 2 significant risk factors for the occurrence of high spinal signal. Cervical instability, as a dynamic damage factor, can inflict repetitive minor traumas on the spinal cord. In the present study, the occurrence rate of ISI of spinal cord was significantly higher in the unstable group than that in the stable group. We speculated that it was related to the long duration of compression of the spinal cord by static protrusion objects and dynamic damage by cervical instability. The relationship between ISI of spinal cord and surgical outcomes is also unclear. Some previous studies reported that the ISI grade reflected the pathological process of the spinal cord injury and the recuperative potentials of the spinal cord [13,16]. Ito et al. [17] reported that ISI grade was correlated with preoperative severity of myelopathy and surgical outcome. Yukawa et al. [18] also found that preoperative ISI on T2-weighted sagittal imaging was correlated with postoperative recovery rate, and they suggested that patients with the greatest ISI had the worst postoperative recovery. However, there several authors expressed different opinions. Matsumoto et al. [19] suggested that the ISI of the spinal cord did not directly affect the efficacy or indicate the severity of the disease condition. Wanda et al. [20] also found that high intensity areas on MRI T2WI were not correlated with the severity of myelopathy or surgical outcomes. Alafifi et al. [21] concluded that a good surgical outcome may be achieved in CSM patients with high intramedullary signal change on T2WI who did not have clonus or spasticity. In the present study, higher occurrence rate of spine cord ISI on MRI T2WI and lower preoperative JOA score were found in the unstable group. However, we did not find any association between ISI of the spinal cord and poor surgical outcomes.
Prognostic factors in CSM with LCI
In this study, the recovery rate of JOA score in the unstable group was lower than in the stable group. Multivariate stepwise logistic regression analysis showed that this may be related to the lower preoperative JOA score, longer duration of symptoms, and more preoperative physical signs in unstable group patients.
The duration of symptoms can reflect the length of the course of CSM, which also influences the progression and severity of myelopathy because of chronic compression by the protrusion objects. Previous studies [22,23] reported that the duration of myelopathy was a significant factor related to the postoperative prognosis of CSM. In the present study, the duration of symptoms in the unstable group was significantly longer than in the stable group. We speculate that this may be related to the pathogenesis of cervical instability in CSM patients. It is widely accepted that both CSM and cervical instability are mainly caused by cervical degeneration, and severe disc degeneration can cause accelerated degeneration of adjacent discs [7]. Eck et al. [24] demonstrated that there can be accelerated degenerative changes and increased stress loading in adjacent segments after anterior cervical fusion surgery. As the degeneration progresses, the CSM disc space is obviously narrowed and there is hyperplasia of facet and Luschka joints. Osteophytes and even bone bridge formation appear on the anterior and/or posterior margin of the vertebral body, and the intervertebral activity obviously decreases and even disappears, which is equivalent to “autofusion”. Based on this “autofusion” in CSM level, there is accelerated degeneration and compensatory increased mobility in other segments, especially in the adjacent segments, which results in cervical segment instability. Thus, we consider that CSM and LCI are different stages of cervical degeneration disease, and LCI occurs after CSM.
The mean number of physical signs in unstable group patients was significantly higher than that in the stable group, and the mean preoperative JOA score was significantly lower in the unstable group. We speculated that this might be related to the longer disease course and complex spinal injury mechanism in CSM patients with LCI. As a dynamic factor, the mechanisms of spinal injury caused by LCI included: (1) Inflammation caused by instability may aggravate the injury of spinal cord; (2) Instability stimulated the sympathetic nerve and caused spinal vessel vasospasm, aggravating spinal injury; and (3) The dynamic stimulation and compression of the spinal cord. In our clinical work, the spinal cord ISI frequently appeared on the unstable segment; however, there was no obvious compression, which might provide direct evidence of spinal cord injury caused by cervical instability. Cervical segmental instability was also an important factor in the pathogenesis of sympathetic cervical spondylosis, and ideal postoperative results can be achieved by surgery of unstable segment fusion treatment [25]. The symptoms of sympathetic cervical spondylosis and direct injury of the spinal cord, both caused by cervical instability, have a direct superposition effect on the symptoms of CSM patients with LCI. In conclusion, the unstable group patients had lower preoperative JOA scores and more physical signs than the stable group patients.
Treatment options for CSM with LCI
Single segmental CSM patients with LCI are not clinically rare. However, it is common that no obvious spinal compression or abnormal signal change of spine cord are observed in unstable segments. Thus, there is a dispute over the necessity of surgical intervention for unstable segments during the operation. Kawakami et al. [11] reported that the unstable cervical segments were not related to the symptoms of cervical spondylopathy and there was no necessity for surgical treatment. However, Katsuura et al. [26] reported that the unstable segment should be fixed if it was above the obviously narrow cervical intervertebral space, even if there were no degenerative changes on MRI. We recommend surgical intervention for unstable segments for CSM patients with LCI in the following circumstances: (1) There were apparent compression objects or obvious ISI of spine cord in unstable segments; (2) There were obvious symptoms of sympathetic cervical spondylosis related to cervical spinal activities; and (3) The instability occurs at the adjacent segment of the mono-segmental CSM. ACDF or ACCF could be used to fuse the unstable segments in the operation. For patients who did not receive surgical intervention for the unstable segments, the duration of time wearing a cervical collar should be appropriately extended after surgery. Lizuka et al. [27] proposed that for patients with apparent cervical instability after cervical open-door laminoplasty, soft collar wearing time should not exceed 4 weeks in order to avoid neck muscle atrophy and axial symptoms. But taking into account the characteristics of the anterior fusion surgery, we suggest that the cervical collar wearing time of should be appropriately extended, but the specific length of time needs further study.
Limitations
There are some limitations to our study. The sample size was small and the follow-up period was short. Studies with larger sample sizes and longer follow-up periods should be performed to confirm our results. We intend to perform further research to explore the correlation between LCI and curvature of the cervical spine.
Conclusions
Patients suffering from CSM with LCI have higher incidence of ISI of the spinal cord on MRI T2WI. Longer duration of symptoms, lower preoperative JOA score, and more preoperative physical signs were highly predictive of a poor surgical outcome for patients of single segmental CSM with LCI.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Fehlings MG, Tetreault LA, Wilson JR, Skelly AC. Cervical spondylotic myelopathy: Current state of the art and future directions. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S1–8. doi: 10.1097/BRS.0b013e3182a7e9e0. [DOI] [PubMed] [Google Scholar]
- 2.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl):190–97S. doi: 10.1016/j.spinee.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176–87. doi: 10.1097/NRL.0b013e3181da3a29. [DOI] [PubMed] [Google Scholar]
- 4.Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: The clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409–21. doi: 10.1177/1073858412467377. [DOI] [PubMed] [Google Scholar]
- 5.Ng HW, Teo EC, Lee KK, Qiu TX. Finite element analysis of cervical spinal instability under physiologic loading. J Spinal Disord Tech. 2003;16(1):55–65. doi: 10.1097/00024720-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi H, Okada K, Hamada M, et al. Etiologic factors of myelopathy. A radiographic evaluation of the aging changes in the cervical spine. Clin Orthop Relat Res. 1987;(214):200–9. [PubMed] [Google Scholar]
- 7.Wang B, Liu H, Wang H, Zhou D. Segmental instability in cervical spondylotic myelopathy with severe disc degeneration. Spine (Phila Pa 1976) 2006;31(12):1327–31. doi: 10.1097/01.brs.0000218508.86258.d4. [DOI] [PubMed] [Google Scholar]
- 8.Dai L. Disc degenerative and cervical instability: Correlation of magnetic resonance imaging with radiography. Spine (Phila Pa 1976) 1998;23(16):1734–38. doi: 10.1097/00007632-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Goel A. Is instability the nodal point of pathogenesis for both cervical spondylotic myelopathy and ossified posterior longitudinal ligament? Neurol India. 2016;64(4):837–38. doi: 10.4103/0028-3886.185379. [DOI] [PubMed] [Google Scholar]
- 10.Matz PG, Anderson PA, Gmff MW, et al. Cervical laminoplasty for the treatment cervical degenerative myelopathy. J Neurosurg Spine. 2009;11(2):157–69. doi: 10.3171/2009.1.SPINE08726. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami M, Tamaki T, Ando M, et al. Preoperative instability does not influence the clinical outcome in patients with cervical spondylotic myelopathy treated with expansive laminoplasty. J Spinal Disord Tech. 2002;15(4):277–83. doi: 10.1097/00024720-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 12.White AA, 3rd, Johnson RM, Panjabi MM, Southwick WO. Biomechanical analysis of clinical stability in the cervical spine. Clin Orthop Relat Res. 1975;(109):85–96. doi: 10.1097/00003086-197506000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Yukawa Y, Kato F, Yoshihara H, et al. MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine (Phila Pa 1976) 2007;32(15):1675–79. doi: 10.1097/BRS.0b013e318074d62e. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno J, Nakagawa H, Inoue T, Hashizume Y. Clinicopathological study of “snake-eye appearance” in compressive myelopathy of the cervical spinal cord. J Neurosurg. 2003;99(2 Suppl):162–68. doi: 10.3171/spi.2003.99.2.0162. [DOI] [PubMed] [Google Scholar]
- 15.Yagi M, Ninomiya K, Kihara M, Horiuchi Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on magnetic resonance imaging. J Neurosurg Spine. 2010;12(1):59–65. doi: 10.3171/2009.5.SPINE08940. [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos CA, Katonis P, Papagelopoulos PJ, et al. Surgical decompression for cervical spondylotic myelopathy: Correlation between operative outcomes and MRI of the spinal cord. Orthopedics. 2004;27(10):1087–91. doi: 10.3928/0147-7447-20041001-19. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Imagama S, Ito K, et al. MRI signal intensity classification in cervical ossification of the posterior longitudinal ligament -predictor of surgical outcomes. Spine (Phila Pa 1976) 2017;42(2):E98–103. doi: 10.1097/BRS.0000000000001717. [DOI] [PubMed] [Google Scholar]
- 18.Yukawa Y, Kato F, lto K, et al. Postoperative changes in spinal cord signal intensity in patients with cervical compression myelopathy: Comparison between preoperative and postoperative magnetic resonance images. J Neurosurg Spine. 2008;8(6):524–28. doi: 10.3171/SPI/2008/8/6/524. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M, Toyama Y, Ishikawa M, et al. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy. Does it predict the outcome of conservative treatment? Spine(Phila Pa 1976) 2000;25(6):677–82. doi: 10.1097/00007632-200003150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Wanda E, Ohmura M, Yonenobu K. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine (Phila Pa1976) 1995;20(20):2226–32. doi: 10.1097/00007632-199510001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. 2007;17(4):315–22. doi: 10.1111/j.1552-6569.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 22.Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: Data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659–66. doi: 10.2106/JBJS.L.01323. [DOI] [PubMed] [Google Scholar]
- 23.Vedantam A, Jonathan A, Rajshekhar V. Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2011;15(6):660–66. doi: 10.3171/2011.8.SPINE11452. [DOI] [PubMed] [Google Scholar]
- 24.Eck JC, Humphrey SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent level intradiscal pressure and segmental motion. Spine (Phila Pa 1976) 2002;27(22):2431–34. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 25.Yu Z, Liu Z, Dang G. Effect of cervical instability in sympathetie cervical spondylosis. Zhonghua Wai Ke Za Zhi. 2002;40(12):881–85. [PubMed] [Google Scholar]
- 26.Katsuura A, Hukuda S, Saruhasi Y, Mori K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J. 2001;10(4):320–24. doi: 10.1007/s005860000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lizuka H, Nakagawa Y, Shimegi A, et al. Clinical results after cervical laminoplasty: Differences due to the duration of wearing a cervical collar. J Spinal Disord Tech. 2005;18(6):489–91. doi: 10.1097/01.bsd.0000154447.83084.b2. [DOI] [PubMed] [Google Scholar]