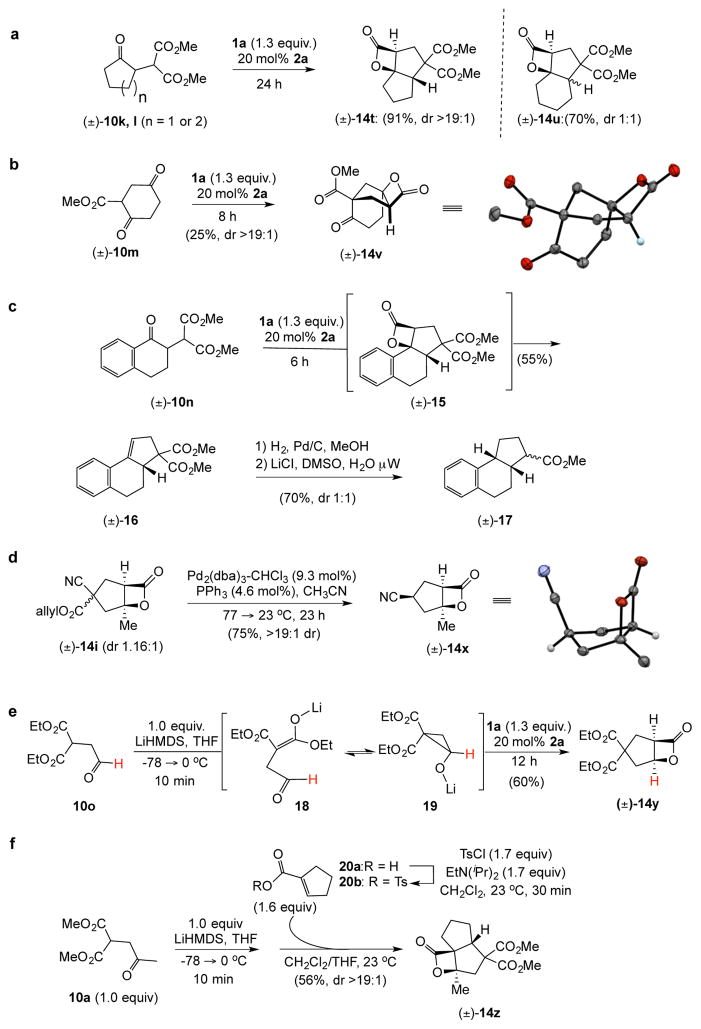
Figure 3. Rapid molecular complexity generation, structural modifications of cyclopentane products, and extensions of both accessible Michael donors and unsaturated acyl ammonium intermediates.
[All NCMAL reactions were performed under standard reaction conditions shown in Table 2 unless noted otherwise.] a, Monocyclic Michael donors with acrylolyl chloride deliver tricyclic 5,5- and 5,6-fused cyclopentyl systems 14t and 14u; b, bridged tricylic cyclopentanes; and c, Truncated steroid intermediates through bis-decarboxylation; d, Mild Pd(0)-mediated reductive decarboxylations leads to cyano substituted cyclopentane 14x. e, Application of aldehyde-containing Michael donors. f, In situ generation of tosyl anhydrides delivers tricyclic 5,5,4-systems from starting carboxylic acids. Relative stereochemistry determined by X-ray analysis (Supplementary, Fig. S8).