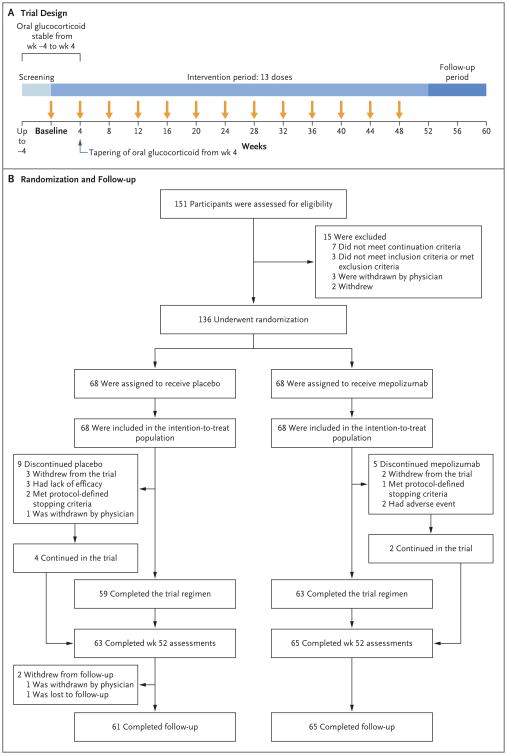
Figure 1. Trial Design and Randomization and Follow-up of the Participants.
The continuation criteria (i.e., the criteria required for undergoing randomization) included the following: glucocorticoid and immunosuppressive therapy stability (the dose had to be stable for ≥4 weeks before randomization); acceptable laboratory assessments, hepatitis status, and liver-function tests (see the Supplementary Appendix); and no clinically significant abnormality on electrocardiography. During the intervention period, participants in the mepolizumab group received 300 mg of mepolizumab plus standard care, and those in the placebo group received matching placebo plus standard care. Mepolizumab or placebo was administered subcutaneously. The intention-to-treat population was used for the primary analysis.