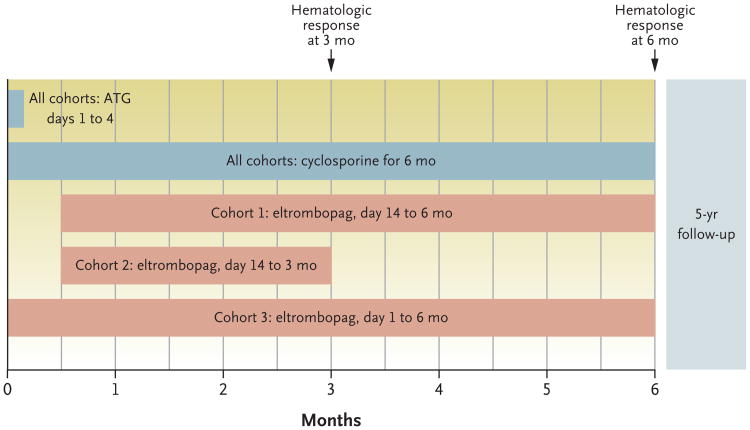
Figure 1. Study Design and Treatment Plan According to Cohort.
All the patients received antithymocyte globulin (ATG) and cyclosporine. Three eltrombopag dosing schemes were implemented in consecutively enrolling cohorts. Results from each cohort informed the design of the subsequent cohort. In cohort 1, eltrombopag was initiated after ATG, owing to concern about overlapping hepatotoxic effects, especially when it is coadministered with ATG and cyclosporine. Since most responses in cohort 1 appeared within 3 months and in order to limit eltrombopag exposure, eltrombopag was discontinued at 3 months in cohort 2. Because the hepatotoxic effects in cohort 2 were found to be infrequent and the rate of complete response was lower than in cohort 1, in cohort 3 eltrombopag was initiated on day 1 with ATG and continued for 6 months. Details regarding the daily dosing scheme are provided in the Supplementary Appendix. The primary end point was the rate of complete hematologic response at 6 months. The rate of partial response and the overall response rate (which included patients with a partial or complete response) were secondary end points.