Figure 1.
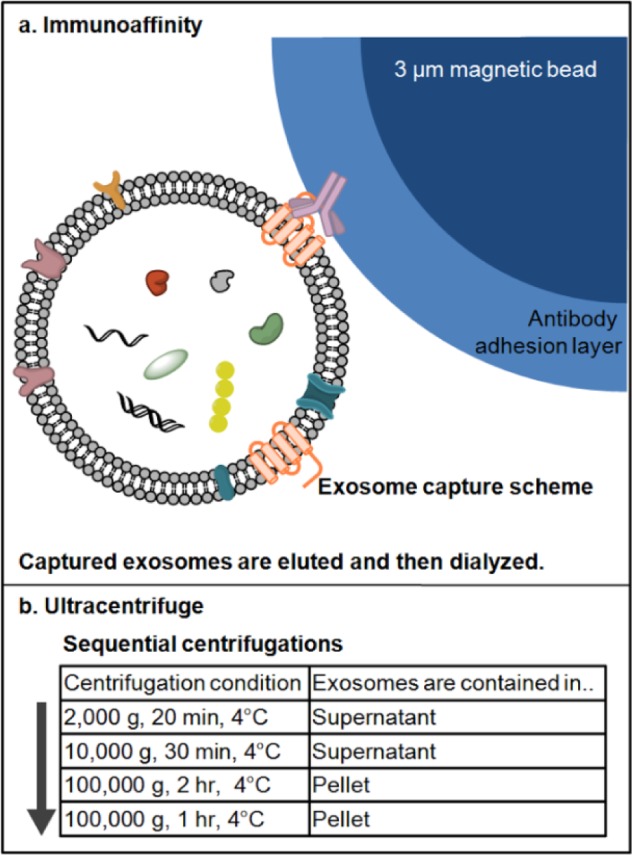
(a) The scheme of exosome isolation by immunoaffinity magnetic beads kit method. For capturing exosomes, antibodies against four different surface markers (CD9, CD63, CD81 and EpCAM) were immobilized on the surface of 3 μm magnetic beads. Each antibody was modified separately on the beads and the mixture of the antibody-beads used to capture antibodies from cell culture media (not drawn in scale). After capture and elution, isolated exosomes were diluted and dialyzed against PBS prior to characterization/storage; (b) flowchart of exosome isolation by ultracentrifugation method. The exosome-containing media was centrifuged sequentially to remove particles denser than exosomes, then centrifuged to pellet down exosomes (exosome buoyant density of 1.10–1.24 g/mL). The flowchart indicates each centrifugation step and the location of exosomes. Exosomes were pelleted following ultracentrifugation at 100 000 g and the pellet was washed with PBS, then ultra centrifuged again. The final exosome pellet was re-suspended in either deionized water or PBS and characterized/stored.
