Abstract
Background
Calpain is a calcium-dependent cysteine protease, and inhibition of calpain by pre-treatment with MDL28170 attenuated the rat mechanical allodynia in a variety of pain models. Postherpetic neuralgia (Shingles) is a neuropathic pain conditioned with the presence of profound mechanical allodynia. Systemic injection of resiniferatoxin can reproduce the clinical symptoms of postherpetic neuralgia. In this study, we determined to study whether activation of calpain contributes to cleave the myelin basic protein of dorsal root and is involved in resiniferatoxin-induced mechanical allodynia of postherpetic neuralgia animal model.
Results
Resiniferatoxin up-regulated the expression and activation of µ-calpain in dorsal root. The expression of µ-calpain was located in Schwann cell of dorsal root, and resiniferatoxin increased the expression of µ-calpain in Schwann cell in L4–L6 dorsal root at six weeks after injection. Resiniferatoxin also induced myelin basic protein degradation in L4–L6 dorsal root at six weeks after injection. Moreover, intraperitoneal injection of calpain inhibitor MDL28170 prevented the degradation of myelin basic protein and then reduced the sprouting of myelinated afferent fibers into spinal lamina II, thus relieving resiniferatoxin-induced mechanical allodynia.
Conclusions
Up-regulation and activation of µ-calpain located in Schwann cell may be the mechanism underlying resiniferatoxin-mediated proteolysis of myelin basic protein in dorsal root. Calpain inhibitor MDL28170 prevents resiniferatoxin-induced sprouting of myelinated afferent fibers and mechanical allodynia through inhibition of degradation of the myelin basic protein in dorsal root. Our results indicate that inhibition of pathological µ-calpain activation may present an interesting novel drug target in the treatment of postherpetic neuralgia.
Keywords: µ-calpain, MDL28170, postherapeutic neuralgia, mechanical allodynia, myelin basic protein, axonal sprouting
Introduction
Calpains, including widely expressed µ-calpain, are intracellular Ca2+-dependent cysteine protease, which limited proteolytic activity, and function to transform or modulate their substrates’ structures and activities; they are therefore called “modulator proteases.”1 Previous studies suggested that calpain is involved in cell death pathways, such as calpain-mediated neuronal injury and thus participate in the pathogenesis of neurodegenerative diseases and degenerative.2 However, the role and mechanism of calpain underlying neuropathic pain are currently poorly understood. Limited studies report that inhibition of calpain by its inhibitors3,4 or silencing of µ-calpain at the spinal level5 attenuates the neuropathic pain following peripheral nerve injury.
Postherpetic neuralgia (PHN, Shingles) is a neuropathic pain with persistent profound mechanical allodynia.6 Previous studies have proved that systemic injection of resiniferatoxin (RTX), an ultrapotent transient receptor potential vanilloid 1 (TRPV1) agonist, can reproduce the clinical symptoms of PHN.7 RTX diminishes the thermal pain sensitivity by depletion of unmyelinated afferent neurons. The delayed tactile allodynia induced by RTX is likely attributable to damage to myelinated afferent fibers and their abnormal sprouting in lamina II of the spinal dorsal horn.7,8 Moreover, the RTX model has been used as a non-viral PHN model to search for the mechanism of neuropathic pain.9,10 Besides, previous studies have shown that TRPV1 activation induces an increase in intracellular calcium and activates Ca2+-activated calpain.11,12 So, we used the RTX-induced PHN model to probe whether activation of calpain contributes to RTX-induced damage of myelinated afferent fibers and the consequent mechanical allodynia.
Myelin basic protein (MBP) is one of the most abundant (30%) myelin proteins and expressed in peripheral nerve by myelination Schwann cells,13 where it mediates myelin sheath thickness, demyelination, and aberrant Schwann cell-axon contacts.14–16 Moreover, previous study has presented extensive degradation of MBP by calpain in traumatic brain injury model,17 and calpain inhibitor blocked nerve injury-induced myelin degradation and neuropathic pain.3 In addition, the studies have reported that µ-calpain but not m-calpain activity mediated proteolysis of myelin and cytoskeletal proteins.18,19 Therefore, we hypothesize that µ-calpain may cleave MBP of dorsal root and be involved in the damage and sprouting of Aβ myelinated afferent fibers in PHN animal model.
In this study, we used an established rat model of RTX-induced PHN to determine whether RTX can activate the calpain and up-regulate the expression of µ-calpain in dorsal root, and then screened the best time point of calpain activation after RTX injection. We also examined the characteristics of distribution of µ-calpain in dorsal root. We then probed whether intraperitoneal injection of calpain inhibitor MDL28170 can prevent myelin breakdown in dorsal root and reduce the sprouting of myelinated afferent fibers into spinal lamina II, thus relieving RTX-induced postherpetic neuralgia.
Methods
Ethics statement
All animal experimental procedures carried out in this study were approved by the Laboratory Animal of the Ethics Committee of Huazhong University of Science and Technology and were in compliance with the guidelines for animal care set forth by this Committee.
Animal models
Experiments were carried out on male adult Sprague-Dawley rats (250–280 g) purchased from Experimental Animal Center of Tongji Medical College of Huazhong University of Science and Technology. All procedures were approved by the Animal Care Committee at Huazhong University of Science and Technology and conformed to the ethical guidelines of the International Association for the Study of Pain.20 The rats were individually housed in cages with a 12-h light/dark cycle and had free access to food and water. Each rat in the RTX group received a single intraperitoneal injection of RTX (250 µg/kg, LC Laboratories, Woburn, MA) under halothane (2% in O2) anesthesia. RTX was dissolved in a mixture of 10% Tween 80 and 10% ethanol in normal saline.21 Each rat in the vehicle group received a corresponding vehicle injection.
Drug administration
Rats were randomly divided into vehicle (vehicle of RTX), RTX, RTX + MDL 28170 treatment, and RTX + PEG 400/DMSO groups. Calpain inhibitor III (MDL 28170) (Calbiochem, Schwalbach/Taunus, Germany) was dissolved in PEG 400/DMSO (1:1) at a concentration of 10 mg/ml. Each rat in the MDL 28170 group was injected intraperitoneally with 25 mg/kg MDL 28170 one week after RTX treatment, once every other day for one week. RTX + PEG 400/DMSO group received an equal volume of vehicle (PEG 400/DMSO (1:1)) injection. Treatments were randomly allocated to animals and the observer was unaware of treatment allocations.22
Nociceptive behavioral tests
To detect mechanical allodynia, we applied von Frey filaments to the left hind paw of rats. Rats were individually placed in suspended chambers on a mesh floor. After the animals were habituated to the testing environment for 30 min, a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) were applied perpendicularly to the plantar surface of left hind paw with sufficient force to bend the filament for 6 s. Brisk withdrawal or paw flinching was considered as a positive response. In the absence of a response, the filament of the next greater force was applied. If there is a response, the filament of the proximal lower force was applied. This up and down procedure was applied four times following the first change in response and stimuli were not re-applied within a 5-min period. The tactile stimulus producing a 50% likelihood of withdrawal response was calculated by using the “up-down” method, as described previously.21,23 The test was repeated two times in each rat, and the mean value was calculated.7
Western blot analysis
To determine the total protein levels in the dorsal root tissues, rats were anesthetized with 10% chloralic hydras (3.5 kg/ml, intraperitoneal) and then decapitated. Six dorsal roots (bilateral L4–L6 dorsal roots) were dissected and homogenized in RIPA lysis buffer with 40 mg/ml (Beyotime Biotechnology, Nanjing, China) and 2 mM phenylmethyl sulfur fluoride and centrifuged at 12,000 g for 15 min at 4℃. The pellet was discarded, and protein concentrations from the supernatant were determined using the enhanced BCA Protein Assay Kit (Beyotime Biotechnology, China). For each sample, 20 µg protein was denatured with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer at 95℃ for 5 min and separated on an 8%–12% glycine-SDS-PAGE gel. The proteins were transferred onto a polyvinylidene fluoride membrane, blocked for 1 h in 5% non-fat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween-20. The membrane was incubated with primary antibodies of rabbit anti-µ-calpain antibody (1:1000, Cell Signaling Technology, Boston, USA); mouse anti-MBP antibody (1:20000, Novus Biological Littleton, CO, USA); mouse anti-α II-spectrin antibody (1:4000, Endo Life Sciences, Farmingdale, NY, USA); or mouse anti-β-actin antibody (1:5000; Santa Cruz, Dallas, TX, USA) at 4℃ overnight. After washing three times for 10 min in 0.1% TBS-Tween 20 (pH, 7.4), the membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies from Santa Cruz Biotechnology (Santa Cruz, USA): goat anti-rabbit secondary antibody (1:20,000) or goat anti-mouse secondary antibody (1:20,000) for 1 h at room temperature, and washed three times for 10 min in 0.1% TBS-Tween 20 (pH, 7.4). The enhanced chemiluminescence method (ECL plus Western blotting detection reagents, Pierce, Rockford, IL, USA) was used to reveal the protein bands according to the protocol of manufacturers. The optical density of each band was then measured with a computer-assisted imaging analysis system (Quantity One, Bio-Rad, UK) and normalized with the housekeeping gene β-actin. Results of independent experiments were expressed as the percentage change over the protein amount in the vehicle group.
Double-immunofluorescence labeling
Rats were deeply anesthetized with 10% chloralic hydras and were transcardially perfused with 37℃ normal saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH7.4; 4℃). Six dorsal roots (bilateral L4–L6 dorsal roots) were quickly removed and post fixed for 2 h in the same fixative solution and cryoprotected in 30% sucrose in 0.1 M phosphate buffer for 48 h at 4℃. The sections were cut at 20 µm on a cryostat, which were mounted onto gelatin-coated slides and air dried overnight.24
The sections were rinsed in 0.1 M PBS and blocked for 1 h with 5% donkey serum and 0.2% Tween-20 in PBS and then incubated with the following primary antibodies at 37℃ for 1 h and at 4℃ overnight: rabbit anti-µ-calpain (1:50, Cell Signaling Technology, Boston, USA); mouse anti-S100 (1:100, Abcam, Hong Kong); and mouse anti-NF-200 (1:500, Sigma, St. Louis, MO, USA). Subsequently, sections were washed four times in PBS for 5 min and incubated with corresponding secondary antibodies from Jackson immune Research (West Grove, PA, USA): donkey anti-rabbit IgG conjugated with Dylight594 (1:400) and donkey anti-mouse IgG conjugated with Dylight 488 (1:400). Sections were washed four times in PBS for 5 min and then coverslipped. Negative controls were included by omitting the primary antibodies and with primary antibodies preabsorbed with their specific blocking peptides in the above procedures, which resulted in no positive labeling in the dorsal root tissues. Digital images were acquired using an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan). Images were captured using a Qimaging Micropublisher RTV 5.0 microscope camera and QCapture Pro 6.0 software (Qimaging, TX, USA). A total of five to six sections from dorsal root were randomly selected in each rat, and counting of single- and double-labeled cells was done on confocal images randomly taken from three view fields from each section. Cell counting was performed by an investigator in a blind fashion using NIH Image J software (Bethesda, MD).
Tracing of myelinated afferent fiber projections to the dorsal horn
The transganglionic tracer, cholera toxin B-subunit (CTB), has been used to map the central projections of cutaneous myelin primary afferents in the spinal dorsal horn of rats.25 After six weeks of RTX injection, CTB (1%, List Biological Laboratories, Campbell, CA) was injected into left sciatic nerve of vehicle-, RTX-, RTX + MDL28170-, and RTX + PEG400/DMSO-treated rats to determine the effect of MDL28170 on RTX-induced sprouting of myelinated afferent fibers labeled by CTB in the dorsal horn. The sciatic nerve was exposed at the level after the rats were anesthetized with 10% chloralic hydras (3.5 ml/kg, intraperitoneal). Four microliters of CTB tracers were loaded into Hamilton microsyringes and injected into the sciatic nerves on the left side of the body.
After allowing four days for transganglionic axoplasmic transport of the tracer, each rat was deeply anesthetized with 10% chloralic hydras and transcardially perfused with 300–400 ml at 37℃ normal saline followed by 500 ml at 4℃ paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The lumbar L4–L6 segment of the spinal cord was quickly removed and post fixed for 2 h in the same fixative solution and cryoprotected in 30% sucrose in 0.1 M phosphate buffer for 48 h at 4℃. The sections were cut (20 µm in thickness) on a cryostat, mounted onto gelatin-coated slides, and air dried. For goat anti-CTB immunofluorescence labeling, the sections were rinsed in 0.1 M PBS and blocked for 1 h with 5% donkey serum and 0.2% Tween-20 in PBS, followed by incubation at 37℃ for 1 h then at 4℃ overnight with the primary antibody (goat anti-CTB, dilution 1:800; List Biological Laboratories, Campbell, CA) diluted in PBS containing 5% donkey serum and 0.3% Triton X-100. Subsequently, sections were washed four times with 0.05% Tween-20 in PBS for 5 min and incubated with a secondary antibody: donkey anti-goat IgG conjugated with Dynight 594 (1:500; Jackson Immune Research). Sections were washed four times with 0.05% Tween-20 in PBS for 5 min, and then coverslipped. An Olympus BX51 fluorescence microscope was used to view the sections, and images were captured using a Qimaging Camera and QCapture software as described before. A total of five to six sections from the spinal cord were randomly selected in each rat. Anatomic outlines of the gray matter of dorsal horn were first plotted and the area of CTB-labeled myelinated afferent fibers in lamina II was measured by using Image J software.
Statistical analysis
Data are presented as means ± SEM. We used one-way analysis of variance (the area of labeled nerve fibers) or two-way analysis of variance (behavioral data) to determine the overall effect of interventions. Post hoc test (Tukey’s or Bonferroni’s) was then used to determine the statistical difference between individual groups. A P value of less than 0.05 was considered statistically significant.
Results
RTX-induced up-regulation of the expression and activation of μ-calpain in L4–L6 dorsal root
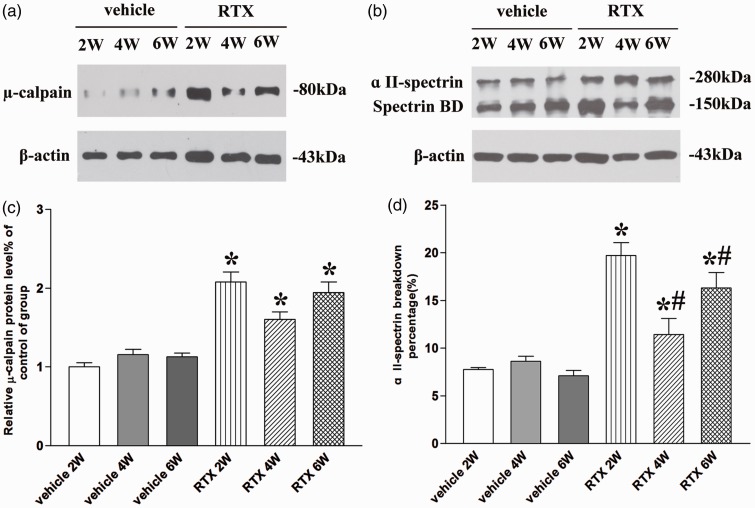
Calpain activation has been reported in neuropathy-related chronic pain conditions.3,5,26 So, we determine the expression and activity of µ-calpain in dorsal root at two, four, and six weeks after RTX injection. The µ-calpain protein expression was present in the dorsal root of vehicle and RTX groups (n = 8, Figure 1(a)). There is no significant difference in the expression level of µ-calpain in dorsal root among two, four, and six weeks after vehicle injection (P > 0.05, Figure 1(c)). But the expression level of µ-calpain was significantly increased in dorsal root tissues at two, four, and six weeks after RTX injection (P < 0.05, Figure 1(c)). Since calpain activated by Ca2+ could be inactivated by self-digestion, direct assessment of tissue calpain activity is difficult.27 However, spectrin is highly sensitive to proteolysis by calpain. The present data report that estimated calpain activity by evaluating the protein level of α II-spectrin breakdown product (150 kDa) using Western blotting.28,29 In this study, we used the percentage of α II-spectrin breakdown product in the total of α II-spectrin to estimate calpain activity. The full-length α II-spectrin and α II-spectrin breakdown product protein expression were present in the dorsal root of vehicle and RTX groups (n = 8, Figure 1(b)). There is no significant difference in the percentage of α II-spectrin breakdown product in the total of α II-spectrin in dorsal root among two, four, and six weeks after vehicle injection (P > 0.05, Figure 1(d)). But the percentage of α II-spectrin breakdown product in the total of α II-spectrin in the dorsal root tissues at two, four, and six weeks after RTX injection was significantly greater than that of vehicle group (P < 0.05, Figure 1(d)). Moreover, the percentage of α II-spectrin breakdown product in the total of α II-spectrin two weeks after RTX injection was significantly higher than that of four and six weeks of injection (P < 0.05, Figure 1(b) and (d)). These data indicate that the best time point of calpain activation is two weeks after RTX injection.
Figure 1.
Quantitative analysis of the expression and activation of µ-calpain in L4–L6 dorsal root. (a) and (b) Representative gel images show the protein level of µ-calpain, α II-spectrin, and α II-spectrin breakdown product (spectrin BD) in L4–L6 dorsal root obtained from vehicle group and RTX group rats. β-actin was used as a loading control. (c) Summary data show the protein level of µ-calpain in L4–L6 dorsal root. (d) Summary data show the percentage of α II-spectrin breakdown product in the total of α II-spectrin in L4–L6 dorsal root. The calpain activity was estimated by evaluating the protein level of α II-spectrin breakdown product (150 kDa) using Western blotting. Data are expressed as means ± SEM (n = 8 rats in each group). *P < 0.05, compared with the vehicle group. *P < 0.05, compared with the RTX group.
RTX-induced MBP degradation in L4–L6 dorsal root at six weeks after injection
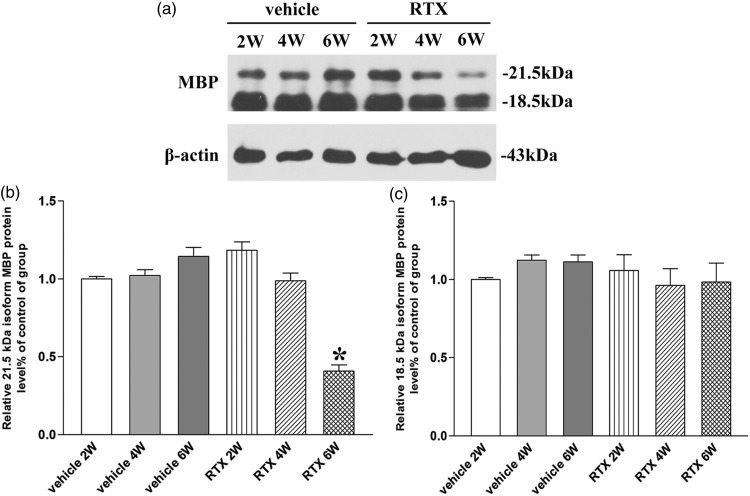
The present data report that the 21.5- and 18.5-kDa MBP isoforms degrade into N-terminal fragments in a rat model of traumatic brain injury.17 We found the 21.5- and 18.5-kDa MBP isoforms total protein expression in dorsal root of vehicle and RTX groups (n = 8, Figure 2(a)). After two, four, and six weeks of injection vehicle, the total protein expression level of 21.5- and 18.5-kDa MBP isoforms had no significant difference compared with vehicle group (P > 0.05, Figure 2(b) and (c)). After two, four, and six weeks of RTX injection, the 21.5-kDa MBP isoform total protein expression was gradually decreased only at sixth week (P < 0.05, Figure 2(b)). But the total protein expression level of 18.5-kDa MBP isoform had no significant difference compared with vehicle group (P > 0.05, Figure 2(c)). These data suggested that RTX induced the 21.5-kDa MBP isoform degradation at six weeks after RTX injection.
Figure 2.
Identification of myelin basic protein (MBP) isoforms degradation in L4–L6 dorsal root. (a) Representative gel images show the total protein level of 21.5- and 18.5-kDa MBP isoforms in L4–L6 dorsal root obtained from vehicle group and RTX group rats. β-actin was used as a loading control. (b) and (c) Summary data show the total protein level of 21.5- and 18.5-kDa MBP isoforms in L4–L6 dorsal root. Data are expressed as means ± SEM (n = 8 rats in each group). *P < 0.05, compared with the vehicle group.
RTX increased expression of µ-calpain in Schwann cell in L4–L6 dorsal root at six weeks after injection
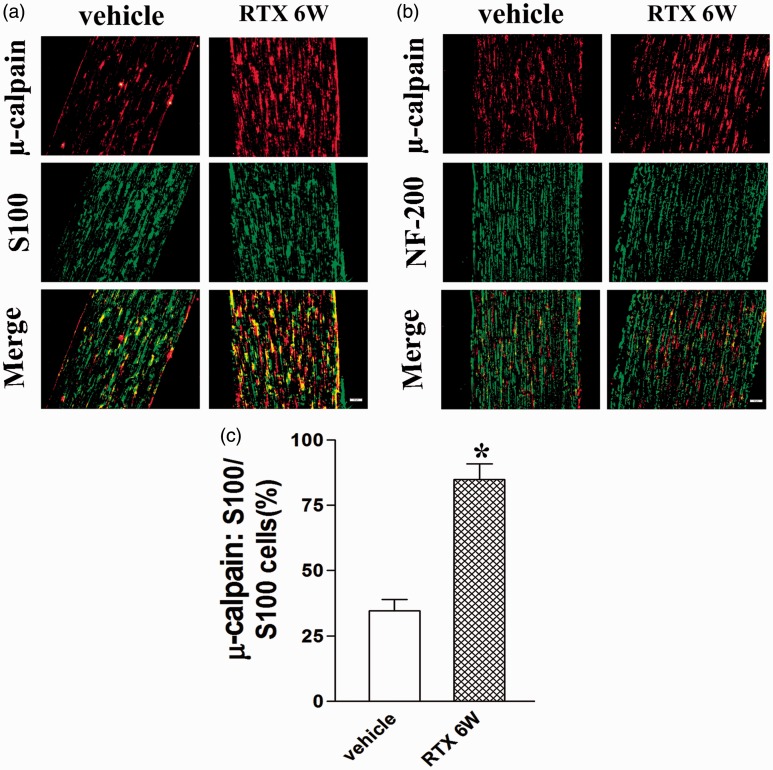
In order to study the characteristics of µ-calpain distribution in dorsal root, we used the Schwann cell marker S100 and the myelinated fibers marker NF-200 to investigate the localization of µ-calpain in dorsal root at six weeks after RTX injection. We found that µ-calpain was coexisted with Schwann cell in L4–L6 dorsal root in both vehicle control and RTX group, but not myelinated fibers (n = 8, Figure 3(a) and (b)). Moreover, RTX up-regulated the number of Schwann cells expressing µ-calpain in L4–L6 dorsal root (P < 0.05, Figure 3(c)). These data suggested that RTX increased the expression of µ-calpain in Schwann cell in L4–L6 dorsal root.
Figure 3.
Double immunolabeling of µ-calpain and Schwann cell marker S100 or NF-200 (marker of the myelinated fibers) in L4–L6 dorsal root. (a) µ-calpain labeling (red); S100 labeling (green); double-labeled cells (yellow). (b) µ-calpain labeling (red); NF-200 labeling (green); no double-labeled yellow cells in merged images. Scale bar, 50 µm. (c) Summary graph show the percentage of double-labeled µ-calpain and S100 immunoreactivity in the total of S100-positive cells in L4–L6 dorsal root from the vehicle and RTX 6W group. Data are expressed as mean ± SEM (n = 8 rats in each group). *P < 0.05, compared with the vehicle group.
Calpain inhibitor MDL 28170 prevented RTX-induced calpain activation and degradation of MBP in L4–L6 dorsal root
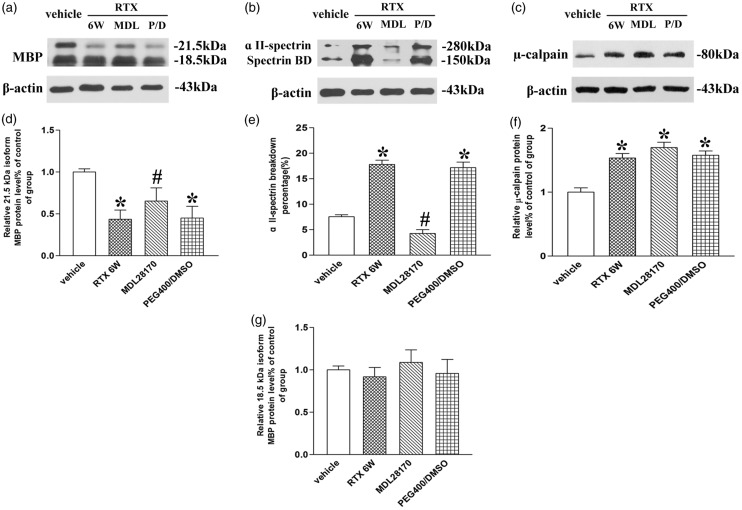
To investigate the potential role of calpain activation in degradation of MBP in L4–L6 dorsal root, MDL 28170 was administered one week after RTX treatment. Rats were randomly divided into vehicle control (vehicle of RTX), RTX, RTX + MDL 28170 treatment, and RTX + PEG 400/DMSO groups (n = 6). The expression level of µ-calpain after MDL 28170 injection had no significant difference with that of vehicle group (P > 0.05, Figure 4(c) and (f)). This is because MDL 28170 only effected the calpain activation.26 The percentage of α II-spectrin breakdown product in the total of α II-spectrin significantly decreased in the dorsal root tissues after MDL 28170 injection (P < 0.05, Figure 4(b) and (e)). The total 21.5-kDa MBP isoform protein expression was gradually increased after MDL 28170 treatment (P < 0.05, Figure 4(a) and (d)). But the total protein expression level of 18.5-kDa MBP isoform after MDL 28170 treatment had no significant difference with that of vehicle group (P > 0.05, Figure 4(a) and (g)). These data illustrated that µ-calpain was contributed to the degradation of 21.5-kDa MBP isoform in L4–L6 dorsal root.
Figure 4.
The effect of calpain inhibitor MDL 28170 on RTX induced calpain activation and degradation of myelin basic protein (MBP) in L4–L6 dorsal root. (a) to (c) Representative gel images show the protein level of µ-calpain, α II-spectrin, α II-spectrin breakdown product (spectrin BD), and 21.5- and 18.5-kDa MBP isoform in L4–L6 dorsal root obtained from vehicle, RTX 6 W, MDL 28170 (MDL), and PEG400/DMSO (P/D) rats. β-actin was used as a loading control. (d), (g), (f) Summary data show the protein level of µ-calpain and 21.5- and 18.5-kDa MBP isoform in L4–L6 dorsal root. (e) Summary data show the percentage of α II-spectrin breakdown product in the total of α II-spectrin in L4–L6 dorsal root. Data are expressed as means ± SEM (n = 6 rats in each group). *P < 0.05, compared with the vehicle group. #P < 0.05, compared with the PEG400/DMSO group.
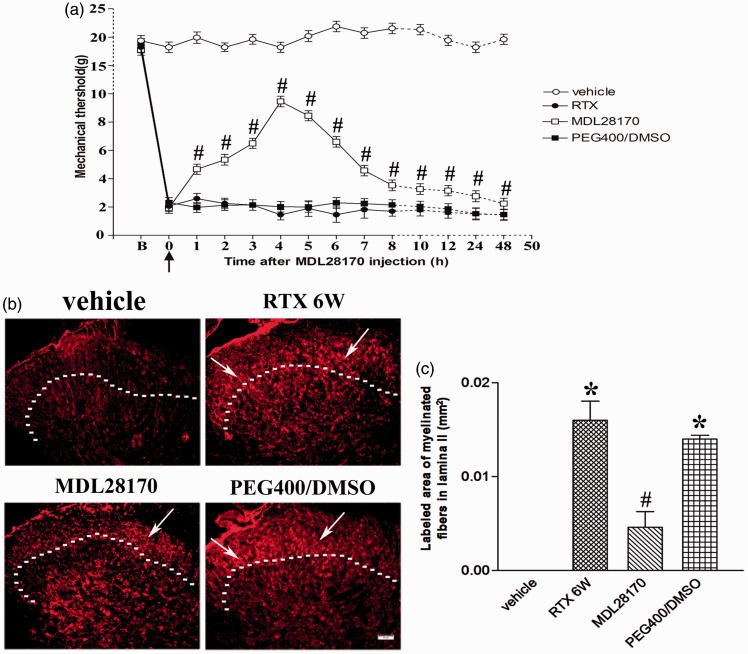
Calpain inhibitor MDL 28170 prevented RTX-induced mechanical allodynia and sprouting of myelinated afferent fibers into the spinal lamina II
Our previous research has found that RTX significantly increased tactile sensitivity and the sprouting of myelinated afferent fibers in the spinal dorsal horn.8 We found that intraperitoneal administration of MDL28170, not PEG400/DMSO, significantly increased the tactile withdrawal thresholds within 48 h after injection (n = 9, P < 0.05, Figure 5(a)).
Figure 5.
The effect of calpain inhibitor MDL 28170 on mechanical allodynia and sprouting of myelinated afferent fibers into the spinal lamina II. (a) Time course of the MDL 28170 effect on RTX-induced mechanical allodynia. The black arrow indicated MDL 28170 injection time; B: baseline. Data are expressed as means ± SEM (n = 9 rats in each group). (b) Representative images show CTB immunoreactive myelinated fibers in the spinal dorsal horn of vehicle, RTX 6W, MDL 28170, and PEG400/DMSO groups. Scale bar, 50 µm. Dotted line marks the division separating lamina II and III. White arrows stands for sprouting of myelinated fibers in lamina II. (c) Summary data show the area of CTB-labeled afferent terminals in lamina II in different groups. Data are expressed as means ± SEM (n = 3 rats in each group). *P < 0.05, compared with the vehicle group. #P < 0.05, compared with the PEG400/DMSO group.
Preferential labeling of myelinated fibers by transganglionic transporting CTB was used to determine whether MDL28170 had any effect on the sprouting of myelinated afferent fibers into the spinal lamina II induced by RTX.8,9 In vehicle-treated group, CTB labeling was quite dense in the medial part of lamina III–V, but was absent in the lamina I–II (n = 3, Figure 5(b)). Some CTB-labeled myelinated afferent fibers sprouted into lamina II in spinal dorsal horn in RTX-treated rats, similar to what we have shown before.8 The area of myelinated fibers labeled by CTB in lamina II of RTX-treated rats was significantly increased than that in the vehicle control group (n = 3, P < 0.05, Figure 5(c)). Intraperitoneal administration of MDL28170, not PEG400/DMSO, significantly attenuated the extension area of myelinated fibers into lamina II (n = 3, Figure 5(b)). The area of CTB-labeled myelinated fibers in lamina II of MDL28170 group was significantly lower than that of PEG400/DMSO group (n = 3, P < 0.05, Figure 5(c)). These data suggested that calpain inhibitor prevents RTX-induced mechanical allodynia and sprouting of myelinated afferent fibers into the spinal lamina II.
Discussion
In the present study, we demonstrated that RTX up-regulated the expression and activation of µ-calpain which located in Schwann cells in dorsal root. RTX also induced MBP (21.5-kDa MBP isoform) degradation in L4–L6 dorsal root at six weeks after injection. Moreover, intraperitoneal injection of calpain inhibitor MDL28170 prevented the degradation of 21.5-kDa MBP isoform in dorsal root, and then reduced the sprouting of myelinated afferent fibers into spinal lamina II, thus relieving RTX-induced mechanical allodynia. Our study provides the first evidence that RTX down-regulates MBP expression in the dorsal root through the µ-calpain activation, thereby causing the sprouting of myelinated afferent fibers into spinal lamina II, then induced mechanical allodynia in RTX-induced PHN animal model.
Calpains, including widely expressed µ-calpain, are Ca2+-dependent neuronal cysteine proteases that are known to be involved in the proteolysis of a number of important proteins, including myelin proteins such as MBP and myelin-associated glycoprotein.2,30 Furthermore, lysophosphatidic acid causes demyelination of the dorsal root through down-regulation of myelin-related proteins, such as MBP, myelin-associated glycoprotein, peripheral myelin protein 22 (PMP22), and myelin protein zero (MPZ) in in vivo injury models and ex vivo culture models.3,15,16 In the present study, we found that RTX up-regulated the expression and activation of µ-calpain and down-regulated MBP protein expression in the dorsal root. Moreover, intraperitoneal administration of calpain inhibitor MDL28170 can prevent the reduction of RTX-induced MBP protein expression. Thus, these findings suggest that RTX induced MBP degradation in the dorsal root in a µ-calpain dependent manner.
For the first time, we found that calpain was activated in dorsal root of RTX-induced PHN model. Calpain is a calcium-dependent cysteine protease requiring calcium ions for its activity.30 Moreover, α II-spectrin is a cytoskeletal protein that is a substrate for the calcium-activated cysteine proteases calpain.31 Calpain cleaves α II-spectrin to generate α II-spectrin breakdown products (145 kDa and 150 kDa)32 and the calpain activity can be estimated by evaluating the protein level of α II-spectrin breakdown product (150 kDa) using Western blotting.29,33 Recent studies have shown that TRPV1 activation induces an increase in intracellular calcium, activates Ca2+-activated calpain, and eventually leads cell injury in diabetic DRG neurons.11,12 Furthermore, RTX may excite TRPV1 receptors and their downstream signaling components Ca2+/calmodulin independent protein kinase and Akt, thus enhance calpain expression and activation in dorsal root of RTX-induced PHN model.34–36
Although calpain is ubiquitously expressed in almost all cells, evidence exists that calpain immune reactivity appeared predominantly in Schwann cell cytoplasm in rat peripheral nerve.37 Consistently, our data illustrate that µ-calpain was coexisted with Schwann cell in dorsal root of RTX-induced PHN model. Moreover, MBP is one of the most abundant (30%) myelin proteins and expressed in peripheral nerve by myelinating Schwann cells.13,38 In addition, previous studies have found that damaged myelinated afferent nerves excessively sprouted into spinal lamina II, which may underlie the mechanism of RTX-induced mechanical allodynia.7,8 Therefore, µ-calpain may promote the degradation of MBP in dorsal root and induced the myelinated fiber damage and lead to the sprouting of myelinated afferent fibers into spinal lamina II.
Previous studies have proved that RTX partially damaged Aβ myelinated afferent, which abnormally (excessively) sprouted into lamina II of the spinal dorsal horn and may underlie the mechanism of mechanical allodynia.8,9 Although the best time point of calpain activation is two weeks after RTX injection, the myelinated fibers did not sprout into lamina II until six weeks after RTX treatment.8 Therefore, we probed whether MDL28170 reduce the sprouting of myelinated afferent fibers into spinal lamina II at six weeks after RTX treatment.
Calpain inhibitor MDL28170 (carbobenzoxy-valylphenylalanial; calpain inhibitor III), which is widely used for study in vivo, readily crosses the blood–brain barrier and cell membranes and has a high specificity for calpain.39,40 Furthermore, previously studies demonstrated that injection of MDL28170 was effective in reducing calpain activity and attenuated neuropathic pain in diabetic neuropathy and L5 ventral root transection model.4,26 Our present study showed that intraperitoneal administration of MDL28170 significantly reduced the calpain activity but did not change the expression of µ-calpain. In addition, intraperitoneal administration of MDL28170 can also prevent the degradation of MBP and the sprouting of myelinated afferent fibers into lamina II of the spinal dorsal horn, and thus relieve RTX-induced mechanical allodynia. MDL28170 is a non-specific calpain inhibitor; therefore, to develop µ-calpain specific inhibitor may have great clinical application value.
Conclusion
In conclusion, up-regulation and activation of µ-calpain located in Schwann cell of dorsal root may be the mechanism underlying RTX-mediated proteolysis of MBP in dorsal root. Furthermore, the calpain inhibitor MDL28170 prevents RTX-induced mechanical allodynia and sprouting of myelinated afferent fibers into spinal lamina II through inhibition of degradation of the MBP. Our results indicate that inhibition of pathological µ-calpain activity may present an interesting novel drug target in the treatment of PHN.
Authors’ Contribution
ML and XCY conceived and designed the experiments. XCY did most of the experiments and analyzed the data. CHW, WY, and FG helped with the western blotting experiment. HCX, HPL and HZ helped with double-immunofluorescence labeling experiments. LXL, WY, and YSL helped with rat model and behavior test experiments. XFM, BT, and XLP helped with the data collection. XCY and ML wrote the manuscript. All authors reviewed the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Natural Science Foundation of China (81473768), and the major project of National Natural Science Foundation of Hubei province (No. 2015CFA094).
References
- 1.Sorimachi H, Hata S, Ono Y. Calpain chronicle—an enzyme family under multidisciplinary characterization. Proc Jpn Acad Ser B Phys Biol Sci 2011; 87: 287–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol 2008; 38: 78–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie W, Uchida H, Nagai J, et al. Calpain-mediated down-regulation of myelin-associated glycoprotein in lysophosphatidic acid-induced neuropathic pain. J Neurochem 2010; 113: 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zang Y, Chen SX, Liao GJ, et al. Calpain-2 contributes to neuropathic pain following motor nerve injury via up-regulating interleukin-6 in DRG neurons. Brain Behav Immun 2015; 44: 37–47. [DOI] [PubMed] [Google Scholar]
- 5.Zhou HY, Chen SR, Byun HS, et al. N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl- cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem 2012; 287: 33853–33864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron R, Saguer M. Postherpetic neuralgia. Are C-nociceptors involved in signalling and maintenance of tactile allodynia? Brain 1993; 116(Pt 6): 1477–1496. [DOI] [PubMed] [Google Scholar]
- 7.Pan HL, Khan GM, Alloway KD, et al. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci 2003; 23: 2911–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CH, Lv ZT, Zhao Y, et al. Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia. Mol Pain 2013; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SR, Pan HL. Effect of systemic and intrathecal gabapentin on allodynia in a new rat model of postherpetic neuralgia. Brain Res 2005; 1042: 108–113. [DOI] [PubMed] [Google Scholar]
- 10.Lei Y, Sun Y, Lu C, et al. Activated glia increased the level of proinflammatory cytokines in a resiniferatoxin-induced neuropathic pain rat model. Reg Anesth Pain Med 2016; 41: 744–749. [DOI] [PubMed] [Google Scholar]
- 11.Hong S, Agresta L, Guo C, et al. The TRPV1 receptor is associated with preferential stress in large dorsal root ganglion neurons in early diabetic sensory neuropathy. J Neurochem 2008; 105: 1212–1222. [DOI] [PubMed] [Google Scholar]
- 12.Chard PS, Bleakman D, Savidge JR, et al. Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience 1995; 65: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 13.Richter-Landsberg C. The oligodendroglia cytoskeleton in health and disease. J Neurosci Res 2000; 59: 11–18. [DOI] [PubMed] [Google Scholar]
- 14.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci 2006; 63: 1945–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue M, Rashid MH, Fujita R, et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 2004; 10: 712–718. [DOI] [PubMed] [Google Scholar]
- 16.Fujita R, Kiguchi N, Ueda H. LPA-mediated demyelination in ex vivo culture of dorsal root. Neurochem Int 2007; 50: 351–355. [DOI] [PubMed] [Google Scholar]
- 17.Liu MC, Akle V, Zheng W, et al. Extensive degradation of myelin basic protein isoforms by calpain following traumatic brain injury. J Neurochem 2006; 98: 700–712. [DOI] [PubMed] [Google Scholar]
- 18.Hinman JD, Duce JA, Siman RA, et al. Activation of calpain-1 in myelin and microglia in the white matter of the aged rhesus monkey. J Neurochem 2004; 89: 430–441. [DOI] [PubMed] [Google Scholar]
- 19.Sloane JA, Hinman JD, Lubonia M, et al. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J Neurochem 2003; 84: 157–168. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 21.Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 2002; 114: 291–299. [DOI] [PubMed] [Google Scholar]
- 22.Kunz S, Niederberger E, Ehnert C, et al. The calpain inhibitor MDL 28170 prevents inflammation-induced neurofilament light chain breakdown in the spinal cord and reduces thermal hyperalgesia. Pain 2004; 110: 409–418. [DOI] [PubMed] [Google Scholar]
- 23.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 24.Wu CH, Yuan XC, Gao F, et al. Netrin-1 contributes to myelinated afferent fiber sprouting and neuropathic pain. Mol Neurobiol 2016; 53: 5640–5651. [DOI] [PubMed] [Google Scholar]
- 25.Woodbury CJ, Ritter AM, Koerber HR. On the problem of lamination in the superficial dorsal horn of mammals: a reappraisal of the substantia gelatinosa in postnatal life. J Comp Neurol 2000; 417: 88–102. [DOI] [PubMed] [Google Scholar]
- 26.Kharatmal SB, Singh JN, Sharma SS. Calpain inhibitor, MDL 28170 confer electrophysiological, nociceptive and biochemical improvement in diabetic neuropathy. Neuropharmacology 2015; 97: 113–121. [DOI] [PubMed] [Google Scholar]
- 27.Manya H, Inomata M, Fujimori T, et al. Klotho protein deficiency leads to overactivation of mu-calpain. J Biol Chem 2002; 277: 35503–35508. [DOI] [PubMed] [Google Scholar]
- 28.Fujisawa H, Numazawa T, Kawamura M, et al. Neurotropin® inhibits calpain activity upregulated by specific alternation of rhythm in temperature in the mesencephalon of rats. Life Sci 2016; 171: 39–44. [DOI] [PubMed] [Google Scholar]
- 29.Roberts-Lewis JM, Savage MJ, Marcy VR, et al. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci 1994; 14: 3934–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev 2003; 83: 731–801. [DOI] [PubMed] [Google Scholar]
- 31.Wang KK, Posmantur R, Nath R, et al. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem 1998; 273: 22490–22497. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Shi Q, Zheng S, et al. Role of alpha-II-spectrin breakdown products in the prediction of the severity and clinical outcome of acute traumatic brain injury. Exp Ther Med 2016; 11: 2049–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujisawa H, Numazawa T, Kawamura M, et al. Neurotropin(R) inhibits calpain activity upregulated by specific alternation of rhythm in temperature in the mesencephalon of rats. Life Sci 2017; 171: 39–44. [DOI] [PubMed] [Google Scholar]
- 34.Talbot S, Dias JP, Lahjouji K, et al. Activation of TRPV1 by capsaicin induces functional kinin B(1) receptor in rat spinal cord microglia. J Neuroinflammation 2012; 9: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han EH, Kim HG, Choi JH, et al. Capsaicin induces CYP3A4 expression via pregnane X receptor and CCAAT/enhancer-binding protein beta activation. Mol Nutr Food Res 2012; 56: 797–809. [DOI] [PubMed] [Google Scholar]
- 36.Kim SR, Kim SU, Oh U, et al. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J Immunol 2006; 177: 4322–4329. [DOI] [PubMed] [Google Scholar]
- 37.Mata M, Kupina N, Fink DJ. Calpain II in rat peripheral nerve. Brain Res 1991; 564: 328–331. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi H, Chattopadhyay S, Kato K, et al. MMPs initiate Schwann cell-mediated MBP degradation and mechanical nociception after nerve damage. Mol Cell Neurosci 2008; 39: 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang KK. Developing selective inhibitors of calpain. Trends Pharmacol Sci 1990; 11: 139–142. [DOI] [PubMed] [Google Scholar]
- 40.Li PA, Howlett W, He QP, et al. Postischemic treatment with calpain inhibitor MDL 28170 ameliorates brain damage in a gerbil model of global ischemia. Neurosci Lett 1998; 247: 17–20. [DOI] [PubMed] [Google Scholar]