Abstract
There is considerable evidence that circadian rhythms in mammals can be modulated by emotional state, but how emotional state modulates specific circadian outputs is poorly understood. We analyzed the expression of the circadian clock protein Period2 (PER2) in three regions of the limbic forebrain known to play key roles in emotional regulation, the central nucleus of the amygdala (CEA), the basolateral amygdala (BLA), and the dentate gyrus (DG). We report here that cells in all three regions exhibit daily rhythms in expression of PER2 that are under the control of the master clock, the suprachiasmatic nucleus (SCN). The rhythm in the CEA and the rhythms in the BLA and DG are diametrically opposite in phase and are differentially affected by adrenalectomy. Adrenalectomy completely abolished the PER2 rhythm in the CEA but had no effect on the PER2 rhythms in the BLA and DG. We previously reported a rhythm in PER2 expression in the oval nucleus of the bed nucleus of the stria terminalis that is identical in phase and sensitivity to adrenalectomy to that found in the CEA. Together, these findings show that key structures of the limbic forebrain exhibit daily oscillations in clock gene expression that are controlled not only by input from the SCN but, importantly, by hormonal and neurochemical changes that normally accompany motivational and emotional states. Thus, cells within these areas are strategically positioned to integrate the inputs from the SCN and emotional states to modulate circadian rhythms downstream from the SCN clock.
Keywords: hippocampus, suprachiasmatic nucleus, oval nucleus, circadian clock
In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus is recognized as a master clock responsible for the regulation of all behavioral and physiological circadian rhythms (1). The rhythm of the SCN, although sensitive to the daily cycle of light, is relatively invulnerable to environmental challenges and changes in physiology (2, 3). Yet it is recognized that stressors and changes in emotional states can have disruptive effects on behavioral and physiological rhythms (4–8). Furthermore, disruptions of circadian rhythms have negative effects on mood and well being, as observed during jet lag or shift work (9, 10).
Although the SCN is the only clock structure required for the generation of circadian rhythmicity, recent studies show that circadian rhythms in expression of clock genes, such as Per1 and Per2, can be found in other brain regions that participate in the control of specific behavioral and physiological outputs. These include, but are not limited to, the striatum, paraventricular hypothalamic nucleus, arcuate nucleus, preoptic area, and olfactory bulbs (11–17). The study of clock genes in these and other functionally defined brain regions may help to determine how the SCN clock controls specific circadian rhythms and, importantly, may contribute to an understanding of how diverse experiences and pathological conditions can affect circadian rhythms downstream from the SCN clock.
We found recently that the clock protein Period2 (PER2) is expressed rhythmically in the oval nucleus of the bed nucleus of the stria terminalis (BNST-OV) (18), a region of the limbic forebrain known to be involved in the regulation of behavioral, autonomic, and endocrine responses to motivationally and emotionally significant stimuli (19, 20). This rhythm was synchronous with the PER2 rhythm in the SCN and was maintained in constant dark. Furthermore, both lesions of the SCN that abolished circadian locomotor activity rhythms and adrenalectomy completely dampened the PER2 rhythm in the BNST-OV. Based on these findings, we concluded that the BNST-OV contains circadian oscillators that could serve as an interface between the output of the SCN clock and emotional and motivational states (18).
The amygdala is another region of the limbic forebrain importantly involved in the control of motivational and emotional states (21–23). Anatomically, the amygdala is subdivided into a number of distinct areas, of which two are particularly important in the regulation of motivational and emotional processes: the basolateral amygdala (BLA) and the central nucleus of the amygdala (CEA) (21, 23–27). In view of our findings in the BNST-OV, it was of interest, therefore, to determine whether these regions of the amygdala might also exhibit daily rhythms of expression of PER2. Furthermore, we assess the expression of PER2 in the dentate gyrus (DG), a region of the hippocampus that is heavily influenced by the BLA (28, 29) and that has been implicated in emotional memory.
Materials and Methods
Animals and Housing. The experimental procedures followed the guidelines of the Canadian Council on Animal Care and were approved by the Animal Care Committee of Concordia University. Adult male Wistar rats (275–300 g) and bilaterally adrenalectomized Wistar rats were purchased from Charles River Breeding Laboratories. All animals had ad libitum access to food and water. Adrenalectomized rats were given saline solution (0.9% NaCl) as a drinking fluid. Rats were housed individually in clear plastic cages equipped with running wheels. The cages were housed in ventilated, sound- and light-tight isolation chambers equipped with a computer-controlled lighting system (VitalView, Mini-Mitter, Sunriver, OR). Wheel-running activity data were recorded with vitalview software (Mini-Mitter) and analyzed with circadia, as described in ref. 18.
Surgery. Rats were anesthetized with a ketamine (100 mg/ml)/xylazine (20 mg/ml) mixture given i.p. (1.5 ml/kg). Electrolytic lesions, aimed at the SCN (1.2 mm posterior to the bregma, 1.9 mm lateral to the midline, and 9.4 mm below the surface of the skull, at a 10° angle) were made by passing 2 mA of current for 15 seconds through stainless steel electrodes (0.28 mm in diameter), insulated except for the tip, using a Grass lesion maker (DC LM5A, Grass Instruments, Quincy, MA). Loss of circadian locomotor activity rhythms in lesioned rats was determined from the wheel-running records obtained over 60 days by using periodgram analysis (circadia).
Tissue Preparation. Rats were injected with an overdose of sodium pentobarbital (100 mg/kg) and were perfused intracardially with 300 ml of cold saline (0.9% NaCl) and then 300 ml of cold 4% paraformaldehyde in a 0.1 M phosphate buffer (pH 7.3). After perfusion, brains were postfixed in 4% paraformaldehyde and stored at 4°C overnight. Serial coronal brain sections (50 μm) were collected from each animal by using a vibratome.
Immunocytochemistry. Free-floating sections were washed in cold 50 mM Tris-buffered saline (TBS) (pH 7.6) and incubated at room temperature for 30 min in a quenching solution made of TBS and 3% (wt/wt) hydrogen peroxide (H2O2). After the quenching phase, sections were rinsed in cold TBS and incubated for 1 h at room temperature in a preblocking solution made of 0.3% Triton X-100 in TBS (Triton-TBS), 3% normal goat serum, and 5% milk buffer. After the preblocking phase, sections were transferred directly into an affinity-purified rabbit polyclonal antibody raised against PER2 (Alpha Diagnostic International, San Antonio, TX) diluted 1:1,000 with a solution of Triton-TBS with 3% normal goat serum in milk buffer. Sections were incubated with the primary antibody for 48 h at 4°C. After incubation in the primary antibody, sections were rinsed in cold TBS and incubated for 1 h at 4°C with a biotinylated anti-rabbit IgG made in goat (Vector Laboratories) diluted 1:200 with Triton-TBS with 2% normal goat serum. After incubation with secondary antibody, sections were rinsed in cold TBS and incubated for 2 h at 4°C with an avidin–biotin–peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories). After incubation with the ABC reagents, sections were rinsed with cold TBS, then rinsed with cold 50 mM Tris·HCl (pH 7.6), and finally rinsed for 10 min with 0.05% 3,3′-diaminobenzidine (DAB) in 50 mM Tris·HCl. Sections were then incubated on an orbital shaker for 10 min in DAB/Tris·HCl with 0.01% H2O2 and 8% NiCl2. After this final incubation, sections were rinsed in cold TBS, wet-mounted onto gel-coated slides, dehydrated through a series of alcohols, soaked in Citrisolv (Fisher), and coverslipped with Permount (Fisher).
Data Analysis. Stained brain sections (14–20 sections per area for each rat) were examined under a light microscope, and images were captured under ×20 magnification by using an XC-77 video camera (Sony, Tokyo), an LG-3 frame grabber (Scion, Frederick, MD), and nih image 1.63 software. Cells immunopositive for PER2 were counted manually on the captured images by using a 400 × 400-μm template for the CEA and BLA and a 200 × 400-μm template for the DG. Within the DG, the template was placed horizontally over the CA3 region. The estimate of the number of PER2 immunoreactive cells per region was calculated from the counts of the 10 images showing the highest number of labeled nuclei unilaterally. Differences between groups were revealed with ANOVA. The significance threshold was set at 0.05 for all analyses.
Results
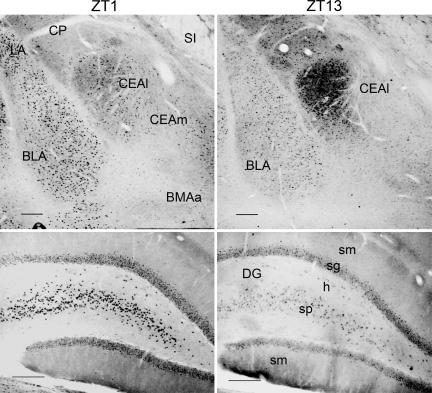
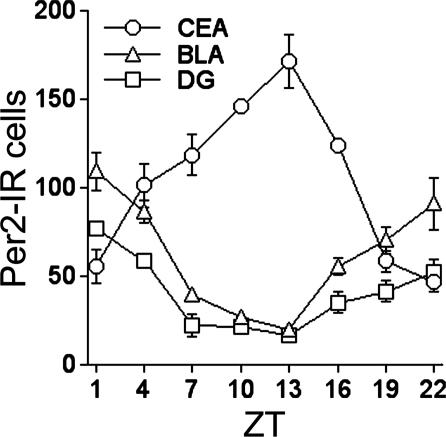
Two Daily Patterns of PER2 Expression. The expression of PER2 in the CEA, BLA, and DG was assessed in rats that were housed under a 12 h:12 h light–dark cycle (300 lux at cage level) for at least 20 days and killed at one of eight equally spaced zeitgeber times (ZTs) (ZT0 denotes time of light on) over the day and night (n = 4 per time point). A significant daily rhythm of nuclear staining for PER2 was seen in each of the three regions, but the time of peak expression differed. In the CEA, PER2 was expressed primarily in the lateral subregion of the nucleus (Fig. 1). Expression was maximal at the beginning of the dark phase of the entraining photocycle (at ZT13) and minimal at ZT1, the onset of the light phase (Figs. 1 and 2). This daily pattern is identical to that seen previously in the BNST-OV and SCN of these same rats (18), demonstrating that the CEA, BNST-OV, and SCN exhibit synchronous rhythms in expression of PER2. In the BLA complex, the distribution of expression was fairly uniform, whereas in the DG, expression was greatest in pyramidal cell layer. In contrast to the CEA, PER2 expression in the BLA and DG was maximal at the beginning of the light phase (at ZT1) and minimal at ZT13 (Figs. 1 and 2). Thus, two synchronous but diametrically opposite rhythms in PER2 expression were revealed, one in the CEA and BNST-OV and the other in the BLA and DG.
Fig. 1.
Expression profiles of PER2 immunoreactivity in the CEA, BLA, and CA3 regions of the DG of rats killed at ZT1 or ZT13. (Scale bars: 200 μm.) CP, caudoputamen; LA, lateral nucleus of the amygdala; SI, substantia innominata; CEAl, central nucleus of the amygdala, lateral part; CEAm, central nucleus of the amygdala, medial part; BMAa, basomedial nucleus of the amygdala, anterior part; sm, molecular cell layer; sg, granular cell layer; h, hilus; sp, pyramidal cell layer.
Fig. 2.
Mean (± SEM) number of PER2-immunoreactive nuclei in the CEA, BLA, and CA3 regions of the DG of rats killed at different ZTs (n = 4 per time point). Cells immunopositive for PER2 were counted manually by using a 400 × 400-μm template for the CEA and BLA and a 200 × 400-μm template for the DG. The number of PER2 immunoreactive cells per region was calculated for each animal from the counts of 10 images showing the highest number of labeled nuclei.
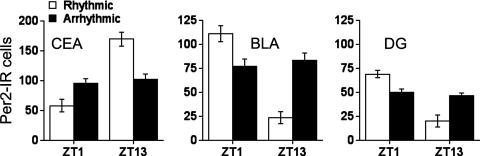
The SCN Controls PER2 Oscillations in the CEA, BLA, and DG. There is considerable evidence that the SCN plays a key role in the regulation of circadian rhythms in Per gene expression in the brain and periphery in rats and mice (11, 13, 30). Furthermore, we reported previously that SCN lesions that abolished circadian locomotor activity rhythms blunted the rhythm in PER2 expression in the BNST-OV, whereas incomplete lesions that were without effect on circadian locomotor activity rhythms had no effect on PER2 expression in the BNST-OV (18). The results of the present analyses were similar; lesions that abolished circadian locomotor activity rhythms abolished PER2 rhythms in the CEA, BLA, and DG, whereas SCN lesions that had no effect on locomotor activity rhythms had no effect on PER2 rhythms in these regions (Fig. 3). These results show that, as is the case with the BNST-OV, the rhythms in PER2 expression in the CEA, BLA, and DG depend on the functional integrity of the SCN clock as determined by its ability to drive circadian locomotor activity rhythms.
Fig. 3.
Mean (± SEM) number of PER2-immunoreactive nuclei in the CEA, BLA, and CA3 regions of the DG of SCN-lesioned rhythmic and SCN-lesioned arrhythmic rats as a function of ZT (n = 6–8 per group).
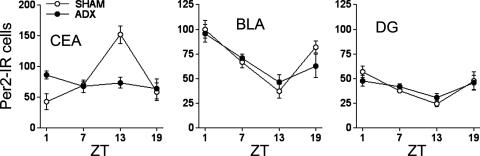
PER2 Oscillation in the CEA, BLA, and DG After Bilateral Adrenalectomy. Cells in the CEA, BLA, and DG express glucocorticoid receptors and exhibit a range of responses to endogenous and exogenous glucocorticoids (31–36). Here, we found that in adrenalectomized rats, the rhythm in PER2 in the CEA was abolished (F[3,18] = 0.91, P = 0.45) (Fig. 4). In contrast, adrenalectomy had no effect on PER2 expression in the BLA and DG. In BLA, PER2 expression was rhythmic in adrenalectomized rats (F[3,18] = 7.34, P = 0.002), and in the DG, the rhythm of expression approached significance (F[3,18] = 2.83, P = 0.06). These rhythms were indistinguishable from those in the BLA and DG of sham operated control rats (Fig. 4). These findings suggest that PER2 expression in these regions is differentially sensitive to glucocorticoids. Our previous findings showing that removal of the adrenal glands blunts the rhythm in PER2 expression in the BNST-OV led us to suggest a role for adrenal glucocorticoid hormones in the control of PER2 oscillations in the BNST-OV (18). The present results show that the rhythm of expression of PER2 in the CEA is similarly controlled by the adrenal gland, whereas that in the BLA and DG are not influenced by adrenal hormones.
Fig. 4.
Mean (± SEM) number of PER2-immunoreactive nuclei in the CEA, BLA, and CA3 regions of the DG of adrenalectomized and sham operated rats as a function of ZT (n = 5–7 per group per time point).
Discussion
The BLA and CEA are known to play distinct but integrative roles in the regulation of emotional state. BLA receives multimodal sensory input from the cortex and thalamus, and studies of phenomena as diverse as fear learning and drug reward suggest that the primary function of the BLA is to integrate information from conditioned and unconditioned stimuli and to communicate this information to areas of the brain involved in motivational and emotional responses (21, 23, 25). A major projection of the BLA is to the CEA, whose primary function is to gate information from the BLA, and from a host of other cortical and subcortical areas, to the limbic-forebrain, hypothalamic, and brainstem regions controlling specific endocrine, autonomic, arousal, and behavioral responses (37–40). Furthermore, there is considerable evidence that information from the BLA is also transmitted to the hippocampus by means of its projections to the entorhinal cortex and can modulate neural plasticity in the DG (41, 42). The BLA modulates neural plasticity in the DG, and this effect has been proposed as a mechanism whereby emotional stimuli affect hippocampal memory processes (28, 29, 43). Several transmitter systems and hormones and a large number of cellular and molecular mechanisms have been proposed to control and modulate neural transmission and plasticity within each of these regions (44). In the present study, we found that cells in the CEA, BLA, and DG exhibit daily rhythms in expression of the clock protein PER2, a key component of the molecular oscillator generating circadian rhythms in cells and tissues of mammals (45, 46). Our finding of circadian oscillations of PER2 in the CEA, BLA, and DG adds a previously unrecognized level of complexity to the control and modulation of activity within these regions.
We identified two temporally distinct patterns of PER2 expression: one in the CEA, where expression peaks in the evening, and one in the BLA and DG, where expression peaks in the morning. Significantly, PER2 expression in the CEA is confined to the lateral part of the nucleus, which closely resembles the cytoarchitecture and chemoarchitecture of the BNST-OV (19, 47–50). Furthermore, the circadian pattern of PER2 expression in the CEA is identical to that which we found previously in the BNST-OV (18). In contrast, the patterns of expression in the BLA and DG resemble those that we have observed in other hippocampal subregions and in cortical areas such as the cingulate, entorhinal, piriform, and insular cortices (data not shown).
The two diametrically opposite rhythms observed in the CEA and BNST-OV and in the BLA and DG were similarly controlled by the SCN clock, as shown by the finding that lesions of the SCN that abolished circadian locomotor activity rhythms also abolished the rhythms of PER2 expression. Indirect projections from the SCN to these regions by means of, for example, the paraventricular nucleus of the thalamus, have been described and could carry circadian information to these structures (51, 52). Loss of PER2 rhythmicity in the CEA, BLA, and DG after functional disruption of the SCN clock suggests that rhythmic expression of PER2 in these structures is driven by the SCN. Alternatively, the SCN may function merely as a synchronizer of rhythms generated in these regions by multiple self-sustaining cellular circadian oscillators (53).
Another important finding, and one with particular significance to the regulation of circadian oscillations within these regions, is that the rhythms in PER2 expression were differentially sensitive to adrenalectomy. As previously reported for the BNST-OV (18), the rhythm in PER2 in the CEA depended on the presence of the adrenal glands, whereas the rhythms in the BLA and DG were unaffected by adrenalectomy. The finding that the PER2 expression in the CEA and BNST-OV is abolished by adrenalectomy suggests that the rhythm of PER2 is modulated by glucocorticoids, which is consistent with evidence that Per gene expression in peripheral tissues is sensitive to glucocorticoids (54, 55). Furthermore, these regions contain high densities of glucocorticoid receptors, and glucocorticoids are known to modulate cell morphology and the expression of peptides such as corticotropin-releasing factor (CRF) and enkephalin (ENK) in these regions (31, 56–62). Preliminary evidence indicates that corticosterone added to the drinking water can restore PER2 rhythms in the CEA and BNST-OV of adrenalectomized, SCN-intact rats. It is important to note, however, that we have yet to determine whether the presence of corticosterone in itself is permissive or whether it is the rhythm of intake that brings about this effect. An additional observation that we made is that cells within the CEA and BNST-OV that express PER2 are immunoreactive to ENK but not to CRF. This observation is interesting in light of findings that ENK cells of the CEA and BNST-OV are particularly sensitive to the effect of stressors and drugs as assessed by c-fos gene expression (63–65).
Contrary to the CEA and BNST-OV, the rhythm of PER2 expression in the BLA and DG was insensitive to adrenalectomy. This finding is surprising, given the overwhelming evidence of the importance of these regions in the effects of glucocorticoids on physiology and behavior. For example, although the BLA has only a moderate level of glucocorticoid receptors, it has been shown that it is critical for the effect of corticosterone on emotional memory (66). The hippocampus contains high levels of glucocorticoid receptors and is well known for its role in stress-induced corticosterone-mediated feedback regulation of the hypothalamic–pituitary–adrenal (HPA) axis (34, 67). Thus, in these regions, there appears to be a dissociation between the effect of glucocorticoids on memory processes and the regulation of the HPA axis and its effects on PER2 expression.
The current view of circadian organization in mammals is that rhythms are controlled by a hierarchical system of circadian oscillators in the brain and periphery that are subordinate to, or enslaved by, the master clock in the SCN (68). Although the function of specific subordinate oscillators is yet to be determined, it is assumed that they serve to gate information from the master clock into tissue-specific rhythmic outputs. In accordance with these ideas, we propose that the cell groups that exhibit rhythms in PER2 expression that we have identified in the amygdala, BNST, and hippocampus are strategically situated to play a role in the circadian modulation of processes normally mediated by these regions. Furthermore, we propose that disturbances of the coupling between the master clock and these cell groups, such as those we have observed after adrenalectomy and after shifts in the entraining light cycle, will affect emotional state and cognitive functioning. Finally, our findings may suggest a mechanism to explain how stressful experiences and changes in emotional state can act downstream from the SCN to affect specific behavioral and physiological circadian rhythms.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, the Fonds de Recherche sur la Nature et les Technologies de Québec, and the Natural Science and Engineering Council of Canada.
Author contributions: E.W.L., J.S., and S.A. designed research; E.W.L. and B.R. performed research; E.W.L., B.R., and S.A. analyzed data; and E.W.L., J.S., and S.A. wrote the paper.
Abbreviations: CEA, central nucleus of the amygdala; BLA, basolateral amygdala; DG, dentate gyrus; SCN, suprachiasmatic nucleus; PER2, Period2; BNST-OV, oval nucleus of the bed nucleus of the stria terminalis; ZT, zeitgeber time.
References
- 1.Rusak, B. & Zucker, I. (1979) Physiol. Rev. 59, 449–526. [DOI] [PubMed] [Google Scholar]
- 2.Challet, E., Caldelas, I., Graff, C. & Pevet, P. (2003) Biol. Chem. 384, 711–719. [DOI] [PubMed] [Google Scholar]
- 3.Hara, R., Wan, K., Wakamatsu, H., Aida, R., Moriya, T., Akiyama, M. & Shibata, S. (2001) Genes Cells 6, 269–278. [DOI] [PubMed] [Google Scholar]
- 4.Gorka, Z., Moryl, E. & Papp, M. (1996) Pharmacol. Biochem. Behav. 54, 229–234. [DOI] [PubMed] [Google Scholar]
- 5.Meerlo, P., van den Hoofdakker, R. H., Koolhaas, J. M. & Daan, S. (1997) J. Biol. Rhythms 12, 80–92. [DOI] [PubMed] [Google Scholar]
- 6.von Zerssen, D., Dirlich, G., Doerr, P., Emrich, H. M., Lund, R. & Ploog, D. (1985) Acta Psychiatr. Belg. 85, 624–635. [PubMed] [Google Scholar]
- 7.Bunney, W. E. & Bunney, B. G. (2000) Neuropsychopharmacology 22, 335–345. [DOI] [PubMed] [Google Scholar]
- 8.Jones, S. H. (2001) Clin. Psychol. Rev. 21, 1193–1209. [DOI] [PubMed] [Google Scholar]
- 9.Cardinali, D. P. (2000) Neuroendocrinol. Lett. 21, 9–15.11455328 [Google Scholar]
- 10.Cho, K. (2001) Nat. Neurosci. 4, 567–568. [DOI] [PubMed] [Google Scholar]
- 11.Abe, M., Herzog, E. D., Yamazaki, S., Straume, M., Tei, H., Sakaki, Y., Menaker, M. & Block, G. D. (2002) J. Neurosci. 22, 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriegsfeld, L. J., Korets, R. & Silver, R. (2003) Eur. J. Neurosci. 17, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto, K., Nagase, T., Fukui, H., Horikawa, K., Okada, T., Tanaka, H., Sato, K., Miyake, Y., Ohara, O., Kako, K. & Ishida, N. (1998) J. Biol. Chem. 273, 27039–27042. [DOI] [PubMed] [Google Scholar]
- 14.Masubuchi, S., Honma, S., Abe, H., Ishizaki, K., Namihira, M., Ikeda, M. & Honma, K. (2000) Eur. J. Neurosci. 12, 4206–4214. [PubMed] [Google Scholar]
- 15.Shieh, K. R. (2003) Neuroscience 118, 831–843. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto, S., Shigeyoshi, Y., Ishida, Y., Fukuyama, T., Yamaguchi, S., Yagita, K., Moriya, T., Shibata, S., Takashima, N. & Okamura, H. (2001) J. Comp. Neurol. 430, 518–532. [PubMed] [Google Scholar]
- 17.Granados-Fuentes, D., Prolo, L. M., Abraham, U. & Herzog, E. D. (2004) J. Neurosci. 24, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amir, S., Lamont, E. W., Robinson, B. & Stewart, J. (2004) J. Neurosci. 24, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong, H. W., Petrovich, G. D., Watts, A. G. & Swanson, L. W. (2001) J. Comp. Neurol. 436, 430–455. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, A. K., de Olmos, J., Pastuskovas, C. V., Zardetto-Smith, A. M. & Vivas, L. (1999) Ann. N.Y. Acad. Sci. 877, 258–280. [DOI] [PubMed] [Google Scholar]
- 21.Cardinal, R. N., Parkinson, J. A., Hall, J. & Everitt, B. J. (2002) Neurosci. Biobehav. Rev. 26, 321–352. [DOI] [PubMed] [Google Scholar]
- 22.See, R. E., Fuchs, R. A., Ledford, C. C. & McLaughlin, J. (2003) Ann. N.Y. Acad. Sci. 985, 294–307. [DOI] [PubMed] [Google Scholar]
- 23.Everitt, B. J., Cardinal, R. N., Parkinson, J. A. & Robbins, T. W. (2003) Ann. N.Y. Acad. Sci. 985, 233–250. [PubMed] [Google Scholar]
- 24.Petrovich, G. D., Scicli, A. P., Thompson, R. F. & Swanson, L. W. (2000) Behav. Neurosci. 114, 681–686. [DOI] [PubMed] [Google Scholar]
- 25.Petrovich, G. D., Risold, P. Y. & Swanson, L. W. (1996) J. Comp. Neurol. 374, 387–420. [DOI] [PubMed] [Google Scholar]
- 26.Sah, P., Faber, E. S., Lopez De Armentia, M. & Power, J. (2003) Physiol. Rev. 83, 803–834. [DOI] [PubMed] [Google Scholar]
- 27.Swanson, L. W. (2000) Brain Res. 886, 113–164. [DOI] [PubMed] [Google Scholar]
- 28.Akirav, I. & Richter-Levin, G. (2002) J. Neurosci. 22, 9912–9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe, K. (2001) Jpn. J. Pharmacol. 86, 18–22. [DOI] [PubMed] [Google Scholar]
- 30.Oishi, K., Sakamoto, K., Okada, T., Nagase, T. & Ishida, N. (1998) Neurosci. Lett. 256, 117–119. [DOI] [PubMed] [Google Scholar]
- 31.Honkaniemi, J., Pelto-Huikko, M., Rechardt, L., Isola, J., Lammi, A., Fuxe, K., Gustafsson, J. A., Wikstrom, A. C. & Hokfelt, T. (1992) Neuroendocrinology 55, 451–459. [DOI] [PubMed] [Google Scholar]
- 32.Conrad, C. D., MacMillan, D. D., II, Tsekhanov, S., Wright, R. L., Baran, S. E. & Fuchs, R. A. (2004) Neurobiol. Learn. Mem. 81, 185–199. [DOI] [PubMed] [Google Scholar]
- 33.Makino, S., Gold, P. W. & Schulkin, J. (1994) Brain Res. 640, 105–112. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto, M., Morita, N., Ozawa, H., Yokoyama, K. & Kawata, M. (1996) Neurosci. Res. 26, 235–269. [DOI] [PubMed] [Google Scholar]
- 35.Roozendaal, B. & McGaugh, J. L. (1997) Neurobiol. Learn. Mem. 67, 176–179. [DOI] [PubMed] [Google Scholar]
- 36.Sousa, N. & Almeida, O. F. (2002) Rev. Neurosci. 13, 59–84. [DOI] [PubMed] [Google Scholar]
- 37.Veening, J. G., Swanson, L. W. & Sawchenko, P. E. (1984) Brain Res. 303, 337–357. [DOI] [PubMed] [Google Scholar]
- 38.Petrovich, G. D. & Swanson, L. W. (1997) Brain Res. 763, 247–254. [DOI] [PubMed] [Google Scholar]
- 39.Pitkanen, A., Stefanacci, L., Farb, C. R., Go, G. G., LeDoux, J. E. & Amaral, D. G. (1995) J. Comp. Neurol. 356, 288–310. [DOI] [PubMed] [Google Scholar]
- 40.Savander, V., Go, C. G., LeDoux, J. E. & Pitkanen, A. (1995) J. Comp. Neurol. 361, 345–368. [DOI] [PubMed] [Google Scholar]
- 41.Nakao, K., Matsuyama, K., Matsuki, N. & Ikegaya, Y. (2004) Proc. Natl. Acad. Sci. USA 101, 14270–14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pikkarainen, M., Ronkko, S., Savander, V., Insausti, R. & Pitkanen, A. (1999) J. Comp. Neurol. 403, 229–260. [PubMed] [Google Scholar]
- 43.Pare, D. (2003) Prog. Neurobiol. 70, 409–420. [DOI] [PubMed] [Google Scholar]
- 44.Pape, H. C. & Stork, O. (2003) Ann. N.Y. Acad. Sci. 985, 92–105. [DOI] [PubMed] [Google Scholar]
- 45.Zheng, B., Larkin, D. W., Albrecht, U., Sun, Z. S., Sage, M., Eichele, G., Lee, C. C. & Bradley, A. (1999) Nature 400, 169–173. [DOI] [PubMed] [Google Scholar]
- 46.Bae, K., Jin, X., Maywood, E. S., Hastings, M. H., Reppert, S. M. & Weaver, D. R. (2001) Neuron 30, 525–536. [DOI] [PubMed] [Google Scholar]
- 47.Cassell, M. D., Gray, T. S. & Kiss, J. Z. (1986) J. Comp. Neurol. 246, 478–499. [DOI] [PubMed] [Google Scholar]
- 48.Ju, G. & Swanson, L. W. (1989) J. Comp. Neurol. 280, 587–602. [DOI] [PubMed] [Google Scholar]
- 49.Ju, G., Swanson, L. W. & Simerly, R. B. (1989) J. Comp. Neurol. 280, 603–621. [DOI] [PubMed] [Google Scholar]
- 50.Dong, H. W., Petrovich, G. D. & Swanson, L. W. (2001) Brain Res. Brain Res. Rev. 38, 192–246. [DOI] [PubMed] [Google Scholar]
- 51.Moga, M. M., Weis, R. P. & Moore, R. Y. (1995) J. Comp. Neurol. 359, 221–238. [DOI] [PubMed] [Google Scholar]
- 52.Peng, Z. C. & Bentivoglio, M. (2004) J. Neurocytol. 33, 101–116. [DOI] [PubMed] [Google Scholar]
- 53.Yoo, S. H., Yamazaki, S., Lowrey, P. L., Shimomura, K., Ko, C. H., Buhr, E. D., Siepka, S. M., Hong, H. K., Oh, W. J., Yoo, O. J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balsalobre, A., Brown, S. A., Marcacci, L., Tronche, F., Kellendonk, C., Reichardt, H. M., Schutz, G. & Schibler, U. (2000) Science 289, 2344–2347. [DOI] [PubMed] [Google Scholar]
- 55.Balsalobre, A., Marcacci, L. & Schibler, U. (2000) Curr. Biol. 10, 1291–1294. [DOI] [PubMed] [Google Scholar]
- 56.Ahima, R. S., Garcia, M. M. & Harlan, R. E. (1992) Brain Res. Mol. Brain Res. 16, 119–127. [DOI] [PubMed] [Google Scholar]
- 57.Schulkin, J., Gold, P. W. & McEwen, B. S. (1998) Psychoneuroendocrinology 23, 219–243. [DOI] [PubMed] [Google Scholar]
- 58.Rosenfeld, P., van Eekelen, J. A., Levine, S. & de Kloet, E. R. (1993) Cell. Mol. Neurobiol. 13, 295–319. [DOI] [PubMed] [Google Scholar]
- 59.Makino, S., Gold, P. W. & Schulkin, J. (1994) Brain Res. 657, 141–149. [DOI] [PubMed] [Google Scholar]
- 60.Watts, A. G. & Sanchez-Watts, G. (1995) J. Physiol. 484, Part 3, 721–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pompei, P., Riftina, F. & McEwen, B. S. (1995) Brain Res. Mol. Brain Res. 33, 209–216. [DOI] [PubMed] [Google Scholar]
- 62.Mulders, W. H., Meek, J., Hafmans, T. G. & Cools, A. R. (1997) Eur. J. Neurosci. 9, 2462–2471. [DOI] [PubMed] [Google Scholar]
- 63.Day, H. E., Curran, E. J., Watson, S. J., Jr., & Akil, H. (1999) J. Comp. Neurol. 413, 113–128. [PubMed] [Google Scholar]
- 64.Day, H. E., Badiani, A., Uslaner, J. M., Oates, M. M., Vittoz, N. M., Robinson, T. E., Watson, S. J., Jr. & Akil, H. (2001) J. Neurosci. 21, 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kozicz, T. (2002) Eur. J. Neurosci. 16, 823–835. [DOI] [PubMed] [Google Scholar]
- 66.Roozendaal, B., Griffith, Q. K., Buranday, J., De Quervain, D. J. & McGaugh, J. L. (2003) Proc. Natl. Acad. Sci. USA 100, 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobson, L. & Sapolsky, R. (1991) Endocrinol. Rev. 12, 118–134. [DOI] [PubMed] [Google Scholar]
- 68.Schibler, U. & Sassone-Corsi, P. (2002) Cell 111, 919–922. [DOI] [PubMed] [Google Scholar]