Abstract
Background
Detection of cholangiocarcinoma (CC) remains a diagnostic challenge particularly in patients with primary sclerosing cholangitis (PSC). We recently established diagnostic peptide marker models in bile and urine to detect CC. Our aim was to combine both models to reach a higher diagnostic accuracy of CC diagnosis.
Methods
Bile (BPA) and urine (UPA) proteome analysis by capillary electrophoresis mass spectrometry was performed in a case-control phase II study on 87 patients (36 CC including 13 with CC on top of PSC, 33 PSC and 18 other benign disorders). A logistic regression model with both analyses was developed and subsequently validated in a prospective cohort of 45 patients.
Results
In the retrospective study, single BPA and UPA showed sensitivities of 83 and 89 % and specificities of 80 and 86 % with an area under the curve (AUC) value of 0.85 and 0.93. If CC was defined as positive UPA and BPA the combination resulted in a sensitivity of 72 % and a specificity of 96 %. The logistic regression model resulted in an increase in sensitivity to 92 % at 84 % specificity with an AUC of 0.96. Applied to the prospective study cohort, the logistic regression model was superior in its sensitivity (94%) and specificity (76%) over single BPA (63% sensitivity, 69% specificity) and UPA (81% sensitivity, 72% specificity) with an AUC of 0.84.
Conclusion
Our logistic regression model enables CC diagnosis with a higher accuracy than currently available diagnostic tools leading potentially to an earlier diagnosis.
Keywords: Cholangiocarcinoma diagnosis, biliary stricture, proteomics, tumor marker, primary sclerosing cholangitis
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown origin eventually leading to end-stage liver disease and cirrhosis. Patients with PSC carry a 160-fold increased risk for cholangiocarcinoma (CC). Therefore, PSC remains the main predisposing factor for CC in Western countries. The detection of CC in patients with PSC remains a diagnostic challenge as the differentiation between malignant and benign strictures is difficult.1 In contrast, early diagnosis of CC in patients with PSC is crucial since early orthotopic liver transplantation (OLT) is the only life-saving therapy and OLT can only be performed in patients with PSC without CC or at an early stage of CC development.
None of the current diagnostic tools is reliable enough to diagnose CC. In the last decade, invasive methods such as endoscopic retrograde cholangiopancreatography (ERCP) with or without cholangioscopy, intraductal ultrasound, brush cytology or forceps biopsies have found their way into clinical practice without having demonstrated a significant impact on the differentiation between malignant and benign biliary lesions.2 Magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) and computed tomography (CT) are valuable tools for the assessment of unclear bile duct lesions. However, the differentiation of malignant bile duct strictures solely through imaging studies is difficult and not feasible.1,2 Furthermore, tumor markers like carbohydrate antigen (CA) 19-9 demonstrated a low sensitivity and specificity in different studies and are therefore not useful to detect CC or for surveillance of patients with risk factors for CC.3
We recently demonstrated that bile and urine proteome analysis (BPA and UPA) have a good diagnostic accuracy to detect CC in patients with PSC.4,5 In this study, our aim was to combine both models to a logistic regression model in order to reach a higher diagnostic accuracy of CC detection compared to single proteome analysis in patients with unknown biliary strictures.
Patients and methods
Patients
Bile and urine samples were collected at the endoscopy unit of Hannover Medical School, Germany. The diagnosis of PSC was based on typical cholangiographic findings such as strictures or irregularity of intrahepatic or extrahepatic bile ducts after exclusion of secondary causes for sclerosing cholangitis. CC was proven histologically. Patients after OLT were excluded from the study. Serological markers were determined by laboratory routine testing. The combined bile and urine test was subsequently evaluated in a prospective analysis of patients who underwent ERCP between 17 April 2012 and 23 April 2015. All patients with benign biliary strictures showed no clinical or imaging signs of CC after six months of clinical follow-up. The trial was approved by the local ethical committee of Hannover Medical School and written informed consent was obtained from all patients.
Sample collection, preparation, and CE-MS analysis
Bile and urine samples collection, preparation and analysis by capillary electrophoresis–mass spectrometry (CE-MS) were performed as previously described.6,7 Midstream urine was collected at the same day of ERCP prior to the intervention.
Statistical analysis
Binomial logistic regression analysis was performed using MedCalc version 11 (Mariakerke, Belgium). Values for sensitivity, specificity, and 95% confidence intervals (CI) were determined by receiver operating characteristic (ROC) analysis with the same software. P values were calculated on the basis of natural logarithm transformed intensities and the Wilcoxon rank sum test.
Results
Bile and urine samples of 36 CC, of whom 13 had CC on top of PSC, 33 PSC, and 18 non-PSC benign biliary stricture patients (10 with choledocholithiasis in the latter group as the most prominent group) collected at the endoscopy unit of Hannover Medical School, Germany between 26 January 2008 and 24 January 2012 were analyzed retrospectively. Extrahepatic CC was present in 69% of patients (25/36) and intrahepatic CC in 31% (11/36), respectively. Distant metastases were identified in 19% of the patients (7/36). The majority of patients had lymph node metastases at diagnosis of CC (22/36; 61%). In 15 patients (15/36; 42%) imaging studies (CT or MRI) showed a suspicious tumor mass (mean tumor size 3.5 cm, range 1–6 cm). In 20 patients (20/36; 56%) only unclear bile duct changes (stenoses or irregularity/thickening of the wall) were detected. One patient had no pathological findings (1/36; 3%). Clinical and demographic data of the patients included in the retrospective training cohort is presented in Table 1.
Table 1.
Clinical and demographic data of the retrospective training and prospective validation cohort of patients that were used for the establishment and validation of a logistic regression model combining bile and urine proteome analysis for cholangiocarcinoma diagnosis.
| Logit model establishment (fitting and bootstrapping) |
Prospective validation |
|||||
|---|---|---|---|---|---|---|
| Study phase | CC | PSC/BBD | p * | CC | PSC/BBD | p * |
| Patient group | ||||||
| Patients, n | 36 | 51 | 16 | 29 | ||
| Type of biliary disease (n) | ||||||
| CC | 23 | 10 | ||||
| CC on-top-of PSC | 13 | 6 | ||||
| PSC | 33 | 28 | ||||
| Choledocholithiasis | 10 | |||||
| Chronic pancreatitis | 3 | |||||
| SSC | 3 | |||||
| CBD dilatation | 2 | 1 | ||||
| Clinical and demographic parameters (normal values) | ||||||
| Age, years, mean/range | 56/25–80 | 52/21–84 | 0.16 | 60/22–85 | 40/23–68 | 0.0001 |
| Female/male, n/n | 10/26 | 14/37 | 1 | 5/11 | 8/21 | 1 |
| Alkaline phosphatase (35–104 U/L), mean/range | 417/96–1023 | 264/47–1475 | 0.0001 | 280/61–483 | 314/88–986 | 0.53 |
| γ-Glutamyltransferase (<39 U/L), mean/range | 474/59–1302 | 247/15–1340 | 0.0004 | 456/10–2212 | 498/27–3638 | 0.53 |
| Bilirubin (<17 µmol/L), mean/range | 159/5–506 | 33/4–225 | <0.0001 | 36/4–176 | 62/4–260 | 0.09 |
| Leucocyte count (4–10/nL), mean/range | 8628/3600–20800 | 6531/2300–11600 | 0.04 | 8160/840–15700 | 8195/1000–20000 | 0.97 |
| C-reactive protein (<8 mg/L), mean/range | 49/2–215 | 17/1–190 | 0.0002 | 23/1–151 | 19/1–91 | 0.9 |
| Alanine aminotransferase (<5 U/L), mean/range | 153/14–716 | 67/16–331 | 0.002 | 94/17–348 | 105/15–548 | 0.86 |
| Aspartate aminotransferase (<32 U/L), mean/range | 139/23–767 | 60/6–378 | <0.0001 | 65/18–186 | 87/16–375 | 0.33 |
| Carbohydrate antigen 19-9 (<27 kU/L), mean/range | 3503/1–81,243 | 68/1–543 | 0.0001 | 619/7–3098 | 84/1–293 | 0.006 |
| Number of detected peptides | ||||||
| in urine, mean/range | 1928/750–3800 | 1876/969–2940 | 0.93 | 2092/877–3116 | 2156/946–3036 | 0.69 |
| in bile, mean/range | 1667/320–4488 | 1899/327–4165 | 0.19 | 1641/751–2792 | 1566/520–3179 | 0.64 |
Two-tailed probability for continuous data and significance level by Fisher exact test for categorical data.
Abbreviations: BBD, benign biliary disorder; CBD, common bile duct; CC, cholangiocarcinoma; PSC, primary sclerosing cholangitis; SSC, secondary sclerosing cholangitis.
BPA and UPA for CC diagnosis: alone and combined
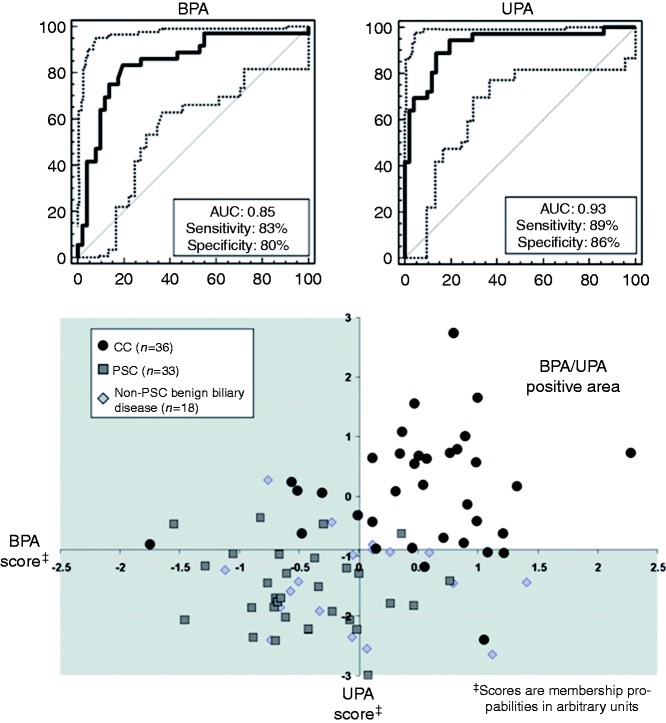
Single BPA showed a sensitivity and specificity of 83% and 80% in the retrospective study cohort of 87 patients (Figure 1(a)). In contrast, UPA displayed a sensitivity and specificity of 89% and 86% in the same set of patients (Figure 1(b)). If both tests were combined by defining CC as a positive test result in both tests a sensitivity of 72% and a specificity of 96% was obtained. The Cartesian graph in Figure 1(c) schematically illustrates the combination of BPA and UPA to this simple majority rule-based model of CC diagnosis. As shown in this figure, all samples in the double-negative lower left quadrant were from patients without CC. In contrast, in the double-positive upper right quadrant only two patients were misclassified as false positive. Information on the peptide marker patterns used for BPA and UPA, especially on the nature of the included naturally occurring peptides, is provided as Supplementary material online.
Figure 1.
Receiver operating characteristic (ROC) curves for classification of the retrospective patient cohort consisting of 36 CC patients of whom 13 have CC on-top-of PSC as case and 33 PSC and 18 non-PSC benign biliary stricture patients as control group by (a) single bile (BPA) and (b) single urine (UPA) proteome analysis. (c) Combination of BPA and UPA for CC diagnosis by a simple majority vote decision model. In this case, CC is defined as a positive test result in both BPA and UPA. In the Cartesian graph, this is true for samples with classification values in the upper right quadrant, designated as BPA/UPA positive area. Contrary, double negative or single positive test results are voted as CC negative.
Establishment of a logistic regression model for CC diagnosis
In order to improve sensitivity of CC diagnosis in patients with bile duct strictures the feasibility of a logistic regression modeling approach was tested by combining BPA and UPA and also by testing the inclusion of classical clinical parameters. Liver transaminases, the tumor marker CA 19-9, the acute phase protein C-reactive protein (CRP), alkaline phosphatase, and bilirubin were included as eligible clinical parameters since they showed statistically significant elevation in their serum levels in the CC case compared to the benign biliary disease control group of the retrospective patient cohort. As shown in Table 2, only bilirubin and CA 19-9 demonstrated acceptable discrimination performance with an AUC above 0.75 for the differentiation of CC patients from those with benign strictures in ROC analysis. This is in line with the finding of a significant difference between CC and PSC patients for CA 19-9 (p = 0.006) and a trend towards it for bilirubin (p = 0.09) also in the prospective study cohort, whereas all other parameters were statistically insignificant (as indicated by the p-values for the group differences in Table 1). Therefore, only these two laboratory parameters were selected for further evaluation. From the respective ROC curves, we defined the value corresponding with the maximum of the Youden index as relevant cutoff point. In the case of bilirubin this was at 43 µmol/L resulting in a sensitivity of 65% and a specificity of 87%, and in the case of CA 19-9 this was at 91 U/L enabling detection of CC with 76% sensitivity and 78% specificity. None of the clinical markers contributes even moderately to the prediction of CC (as revealed by regression coefficients close to zero and odds ratios close to one) when all of them were combined in multivariate logistic regression analysis (Table 2).
Table 2.
Multivariate logistic regression analysis of relevant clinical variables for prediction of CC in the 87 patients of the retrospective study cohort. As shown, the regression coefficients of all clinical variables in multivariate logistic regression analysis are close to zero and the odds ratios with their 95% CIs are close to 1, which indicates that none of these variables in concert significantly contributes to the prediction of CC.
| Clinical variable | Univariate ROC analysis |
Multivariate logistic regression analysis |
|
|---|---|---|---|
| AUC [95% CI] | Regression coefficient# | Odds ratio [95% CI]‡ | |
| Alkaline phosphatase | 0.71 [0.64–0.78] | −0.008 | 0.99 [0.98–1.00] |
| γ-Glutamyltransferase | 0.68 [0.60–0.75] | 0.006 | 1.01 [1.00–1.01] |
| Bilirubin | 0.76 [0.69–0.83] | 0.018 | 1.02 [1.00–1.04] |
| C-reactive protein | 0.71 [0.64–0.78] | 0.005 | 1.00 [0.99–1.02] |
| Alanine aminotransferase | 0.66 [0.59–0.73] | 0.005 | 1.00 [0.98–1.03] |
| Aspartate aminotransferase | 0.73 [0.65–0.79] | 0.022 | 1.02 [0.98–1.06] |
| Carbohydrate antigen 19-9 | 0.82 [0.73–0.88] | 0.001 | 1.00 [1.00–1.01] |
Abbreviations: CI, confidence interval; ROC, receiver operating characteristic.
Regression coefficient expresses the coefficient with which the variable is multiplied in the regression equation
Odds ratio expresses the amount of change in the logistic regression equation by one unit change in the variable.
Logistic regression analysis was subsequently performed using clinical diagnosis (absence/presence) of CC as the dependent binary variable and the classification factors of BPA and UPA and serum levels of CA 19-9 and bilirubin as independent variables. Analysis of CA 19-9 and bilirubin showed no statistical significance for contribution to the diagnostic decision (see Table 3 for correlation coefficients and significance values of all tested independent variables) and were therefore excluded from the calculation of the logistic regression (logit) function.
Table 3.
Multiparameter logistic regression analysis of proteome and clinical variable contributions to the diagnosis of CC in the retrospective 87-patient cohort. For normalized CA 19-9 and bilirubin serum levels, it was tested if they significantly contribute to CC diagnosis when added to the logistic regression model of combined bile and urine proteome analysis.
| Independent variable† | Multivariate logistic regression analysis |
||
|---|---|---|---|
| Regression coefficient ± SE | Odds ratio‡ [95% CI] | Significance level (p) | |
| Bile proteome analysis (BPA) | 1.83 ± 0.62 | 6.25 [1.87–20.94] | 0.003 |
| Urine proteome analysis (UPA) | 2.64 ± 0.67 | 13.96 [3.73–52.22] | 0.0001 |
| Logit BPA/UPA regression model | 6.17 ± 1.63 | 479.16 [19.59–11718.10] | 0.00015 |
| +CA19-9 | 4.48 ± 12.53 | 32.56 [0.00–1.49E + 12] | 0.78 |
| + bilirubin | 1.07 ± 1.17 | 2.91 [0.29–28.66] | 0.36 |
Abbreviations: CI, confidence interval; ROC, receiver operating characteristic; SE, standard error.
All clinical variables were normalized by subtraction of the mean and division by the standard deviation.
Regression coefficient expresses the coefficient with which the variable is multiplied in the regression equation.
Odds ratio expresses the amount of change in the logistic regression equation by one unit change in the variable.
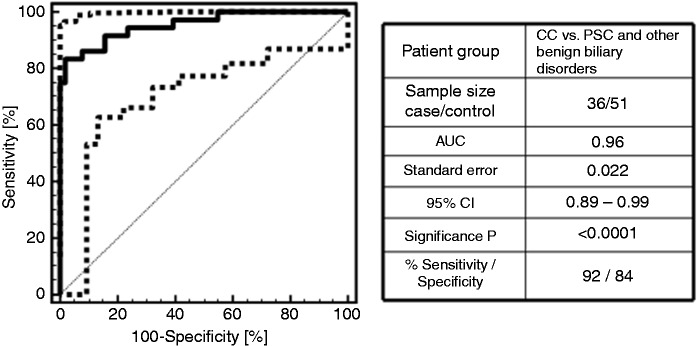
Despite the serological markers, the classification factors of the bile and urine proteome model were significant predictors of clinical diagnosis of CC with odds ratios of 6.2 (95% CI: 1.9–20.9) for BPA and 14.0 (95% CI: 3.7–52.2) for UPA. A regression equation was calculated with the regression coefficients for the BPA and UPA classification factors as independent variables. Based on the estimated correlation coefficients of 1.8325 for BPA and 2.6363 for UPA the regression equation reads as follows: BPA/UPA match = 1.6128 + 1.8325 * BPA score + 2.6363 * UPA score with the BPA/UPA match representing a probability score for the presence of CC. Since the correlation coefficients express the change in the logged odds of having CC, more weight is given to a positive test result in UPA than BPA. A ROC analysis performed on the logit function of BPA and UPA classification factors for the 36 CC case and 51 PSC and other patients with benign biliary disorders revealed 96% test accuracy with 92% sensitivity and 84% specificity at the best probability cutoff at 0.52. The ROC curve for the logit UPA/BPA classification model is presented in Figure 2. The logit classification model showed a difference in AUC of 0.11 compared to BPA (p = 0.009) and 0.03 compared to UPA (p = 0.112). In relation to the diagnosis based on a positive test result in both the BPA and UPA test, the logit regression function significantly improves sensitivity (92% compared to 72%, increase of 20%) of CC diagnosis without significant loss of specificity (84% compared to 96%, decrease of 12%). Here, sensitivity and specificity values for the two different bile and urine proteome combinatory models were calculated by the ratio of true positives and the sum of true positives and false negatives in the case of sensitivity and by the ratio of true negatives and the sum of true negatives and false positives in the case of specificity. For testing of significance, a student’s t-test was applied to the numbers of true or false positive or negative observations between the two models.
Figure 2.
Receiver operating characteristic (ROC) curve for the diagnosis of cholangiocarcinoma (CC) and its discrimination from primary sclerosing cholangitis (PSC) and other benign biliary disorders by the logistic regression model. The regression model showed an increase in the area under ROC curve (AUC) of 0.11 compared to bile proteome analysis (p = 0.009) and 0.03 compared to urine proteome analysis (p = 0.112) by improving sensitivity of CC diagnosis at the same level of specificity in comparison to the single proteomic tests presented in Figure 1(a) and (b).
Prospective validation of the BPA/UPA logistic regression model for CC diagnosis
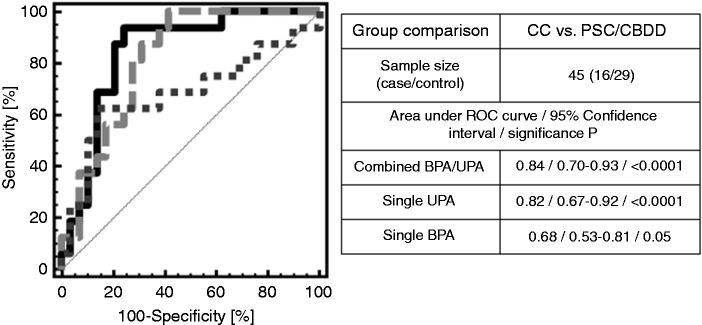
Since the regression model was established using a logistic fitting algorithm to identify the best correlation values included in the logistic regression formula, it is mandatory according to recent guidelines for the conduct of clinical proteomic studies to validate a multivariate diagnostic pattern on an independent set of patient samples. This was performed for a set of patients enrolled between 17 April 2012 and 23 April 2015 in prospective analysis for which a well-established clinical outcome was later available. This account for 16 CC cases, including 6 with CC on-top-of PSC, and 29 non-CC controls, 28 with PSC and one with common bile duct dilatation (CBDD), which can be transduced to a CC prevalence in the prospective cohort of 36%. The patients with CC had extrahepatic manifestation in 88% (14/16) and intrahepatic manifestation in 13% (2/16), respectively. Liver metastases were present in 13% (2/16) of patients. No other distant metastases were identified. In four patients (4/16; 25%) imaging studies (CT or MRI) showed a suspicious tumor mass at the level of the bifurcation of the bile ducts (mean tumor size 2.1 cm, range 1–3 cm). In the other patients (10/16; 63%) only unclear bile duct changes (stenoses or irregularity/thickening of the wall) were detected. Two patients (2/16; 13%) had no pathological findings. As presented in Figure 3, classification by the BPA/UPA logistic regression model resulted in an AUC of 0.84 (p < 0.0001) and a 95% CI in the range 0.70–0.93 in ROC analysis. At the cutoff of >−0.5 that was predetermined during establishment of the logistic regression model a sensitivity of 94% (95% CI: 70–100 %), a specificity of 76% (95% CI: 57–90 %), and negative and positive predictive values of 96% (95% CI: 78–100 %) and 68% (95% CI: 45–86 %) were determined on this prospective set of patient samples. In fact, only one patient in the prospective cohort with later histologically confirmed CC in situ after explorative laparotomy had a false negative test result. Besides the negative proteomic test result, no elevation in CA19-9 serum levels and no signs of tumor in endoscopic ultrasound and MRI could be detected for this patient.
Figure 3.
Classification of the prospective patient cohort consisting of 28 patients with primary sclerosing cholangitis (PSC) and one patient with common bile duct dilatation (CBDD) as negative group and 16 cholangiocarcinoma (CC) (including six with CC on-top-of PSC) patients as positive group by the BPA/UPA logistic regression model in comparison to single bile (BPA) or urine (UPA) proteome analysis.
The combined BPA/UPA test resulted in a significant improvement in test accuracy compared to single BPA analysis (AUC: 0.68, sensitivity and specificity of 63 and 69 % at the predetermined cutoff of 0.01) on the same set of patients (difference in the AUC’s of 0.16).4 In comparison to single UPA analysis (AUC: 0.82, sensitivity and specificity of 81 and 72 % at the predetermined cutoff of −0.89),5 the difference in the AUCs is only marginal (0.02), but inclusion of BPA in this case results in a shift towards a higher sensitivity (94% versus 81%) at the same level of specificity (76% versus 72%).
Discussion
It still remains unknown, if and when a patient with PSC develops CC. Therefore, the main focus of clinicians is to detect CC as early as possible. Unfortunately, the differentiation between benign and malignant bile duct strictures in PSC is particularly difficult, because CC as well as chronic or acute inflammation may result in similar cholangiographic findings.2 Due to the grim prognosis of CC innovative diagnostic tools are urgently needed to improve the detection of CC in populations at risk.8,9
Clinical and laboratory parameters are also of limited value for the discrimination of unclear bile duct strictures. For instance, elevated transaminases, cholestatic parameters and bilirubin may reflect hepatobiliary inflammation but do not play a role in the detection of biliary malignancy. In fact, bilirubin which is often raised in CC is not sensitive, as it can be dramatically increased in benign strictures such as PSC or biliary stones.10 Moreover, the tumor marker CA 19-9 is not expressed by 5–10 % of the population (leading to false negative test results) and is also frequently raised in patients with benign biliary obstruction and therefore is not considered as a sensitive marker in clinical practice.10
A straightforward approach for accurate detection of CC is the identification of markers in bile, as the development of carcinoma takes place at the biliary epithelium and tumor-related proteins are secreted or shed into the bile. BPA was successfully performed leading to 84% sensitivity and 78% specificity in discriminating CC from PSC in a validation cohort of 25 CC and 18 PSC patients.4
In a recent study, we established a peptide marker model based on urinary peptides that mirror systemic effects of CC tumor progression.5 The urine proteomic model was proven to be of equal diagnostic precision as that in bile. Based on UPA we were able to detect CC in 35 of 42 patients (83% sensitivity) and to exclude it in 64 of 81 patients (79% specificity) with definite histology in cross-sectional validation. In fact, the urine peptide marker model identified CC in all patients with CC on-top-of PSC, indicating its potential for PSC surveillance.
Our new data indicate that the combination of BPA and UPA in a logistic regression model has even a higher diagnostic accuracy compared to both tests alone. The advantage of the combined bile and urine proteomic test is its high specificity (only two false positive classifications out of 51 patients with benign strictures) if CC is defined in a majority vote approach as a positive result in both tests. From the clinical point of view, a high specificity is important to avoid unnecessary surgery as surgery may be associated with 5-10% mortality and even with margin-free resection 5-year survival rates only reach 20–40%.8,9 However, a sensitivity of 72% in the case when the presence of CC is defined as a positive test result in both the BPA and UPA test is still not markedly higher than the reported sensitivity of imaging techniques and endoscopic interventions.11–17 This implicates the need for a complimentary test with the trait of a high sensitivity.
We found that the combination of the classification scores for BPA and UPA to a logistic regression model improves sensitivity of CC diagnosis from 72 to 94 %. This gain in sensitivity by combined BPA and UPA analysis is required for reliable diagnosis of CC in patients with unclear biliary strictures. Furthermore, surveillance of patients with PSC during their time on the waiting list for OLT with the highly sensitive CC BPA/UPA test may be of considerable diagnostic value, since it may allow periodical screening for signs of CC with the non-invasive UPA test. In case of a positive test result a follow-up with an ERCP including brush cytology, forceps biopsy, and bile aspiration for BPA analysis may be performed. In this respect, a negative predictive value of 0.88 was calculated for single UPA on the prospective set of PSC patients used in this study. These findings suggest that the UPA may be a valuable tool during PSC surveillance.
The fact that the high diagnostic accuracy of the CC BPA/UPA test could be validated prospectively in an unselected target group of patients indicates that the CE-MS-based test may be of potential diagnostic value to diagnose CC in clinical routine. Since the CE-MS technology has proven to meet all analytical requirements for diagnostic systems, as already demonstrated in a recent technical report and large-scale prospective and/or longitudinal clinical studies,18–22 no transfer of the CC BPA/UPA test to another analytical platform is required for the use of the proteomic test in a routine clinical setting.
One limitation of the present study is that the demonstration of increased sensitivity of the combined bile and urine test is based on a restricted number of 45 prospectively collected patient specimens. Consequently, a separate analysis of CC development in patients with PSC is not feasible. Our future aim will be to evaluate the potential of BPA and UPA in a prospective multicenter clinical study on larger patient cohorts under the following case specific objectives. Firstly, to confirm further that the combined bile and urine test for CC detection significantly improves the diagnostic precision of ERC-based diagnosis in patients with suspicion of CC. Secondly, to determine how early CC can be detected in patients with progressive cholestasis under CC surveillance by regular non-invasive UPA screening.
In summary, our data indicate that complimentary use of combined proteomic analysis of bile and urine improves CC diagnosis in patients with biliary strictures of unknown origin. A large prospective trial also including longitudinal Cox proportional hazards regression analysis on patients with progressive cholestasis during surveillance is required to confirm accurate and early diagnosis of CC. Proofing this will impact the management of PSC patients with increased risk of CC insofar as preventive strategies, curative treatment, i.e. OLT, or future CC antitumor therapies can be applied in time and thus more effectively.
Declaration of conflicting interests
Harald Mischak is founder and co-owner of Mosaiques Diagnostics GmbH, which developed the CE-MS technology and the MosaiquesVisu software. Jochen Metzger is employee of Mosaiques Diagnostics GmbH. All other authors have no financial or nonfinancial competing interests to declare.
Funding
This work was supported by a grant from PSC-Partners – Seeking a Cure to Tim Lankisch.
Supplemental Material
Supplementary material for this paper can be found at http://journals.sagepub.com/doi/suppl/10.1177/2050640616687836
References
- 1.Weismüller TJ, Lankisch TO. Medical and endoscopic therapy of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol 2011; 25: 741–752. [DOI] [PubMed] [Google Scholar]
- 2.Weismüller TJ, Wedemeyer J, Kubicka S, et al. The challenges in primary sclerosing cholangitis: aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol 2008; 48: S38–S57. [DOI] [PubMed] [Google Scholar]
- 3.Sinakos E, Saenger AK, Keach J, et al. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol 2011; 9: 434–439. [DOI] [PubMed] [Google Scholar]
- 4.Lankisch TO, Metzger J, Negm AA, et al. Bile proteomic profiles differentiate cholangiocarcinoma from primary sclerosing cholangitis and choledocholithiasis. Hepatology 2011; 53: 875–884. [DOI] [PubMed] [Google Scholar]
- 5.Metzger J, Negm AA, Plentz RR, et al. Urine proteomic analysis differentiates cholangiocarcinoma from primary sclerosing cholangitis and other benign biliary disorders. Gut 2013; 62: 122–130. [DOI] [PubMed] [Google Scholar]
- 6.Negm AA, Schott A, Vonberg RP, et al. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest Endosc 2010; 72: 284–291. [DOI] [PubMed] [Google Scholar]
- 7.Metzger J, Kirsch T, Schiffer E, et al. Urinary excretion of twenty peptides forms an early and accurate diagnostic pattern of acute kidney injury. Kidney Int 2010; 78: 1252–1262. [DOI] [PubMed] [Google Scholar]
- 8.Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis 2004; 24: 165–175. [DOI] [PubMed] [Google Scholar]
- 9.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology 2003; 37: 961–969. [DOI] [PubMed] [Google Scholar]
- 10.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012; 61: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 11.Voigtländer T, Lankisch TO. Endoscopic diagnosis of cholangiocarcinoma: From endoscopic retrograde cholangiography to bile proteomics. Best Pract Res Clin Gastroenterol 2015; 29: 267–275. [DOI] [PubMed] [Google Scholar]
- 12.Stavropoulos S, Larghi A, Verna E, et al. Intraductal ultrasound for the evaluation of patients with biliary strictures and no abdominal mass on computed tomography. Endoscopy 2005; 37: 715–721. [DOI] [PubMed] [Google Scholar]
- 13.Tischendorf JJ, Meier PN, Schneider A, et al. Transpapillary intraductal ultrasound in the evaluation of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Scand J Gastroenterol 2007; 42: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 14.Levy MJ, Baron TH, Clayton AC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol 2008; 103: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trikudanathan G, Navaneethan U, Njei B, et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 2014; 79: 783–789. [DOI] [PubMed] [Google Scholar]
- 16.De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 1). Gastrointest Endosc 2002; 56: 552–561. [DOI] [PubMed] [Google Scholar]
- 17.De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc 2002; 56: 720–730. [DOI] [PubMed] [Google Scholar]
- 18.Mischak H, Vlahou A, Ioannidis JP. Technical aspects and inter-laboratory variability in native peptide profiling: the CE-MS experience. Clin Biochem 2013; 46: 432–443. [DOI] [PubMed] [Google Scholar]
- 19.Weissinger EM, Metzger J, Dobbelstein C, et al. Proteomic peptide profiling for preemptive diagnosis of acute graft-versus-host disease after allogeneic stem cell transplantation. Leukemia 2014; 28: 842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Good DM, Zurbig P, Argiles A, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics 2010; 9: 2424–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zürbig P, Jerums G, Hovind P, et al. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes 2012; 61: 3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein J, Lacroix C, Caubet C, et al. Fetal urinary peptides to predict postnatal outcome of renal disease in fetuses with posterior urethral valves (PUV). Sci Transl Med 2013; 5: 198ra06–198ra06. [DOI] [PubMed] [Google Scholar]