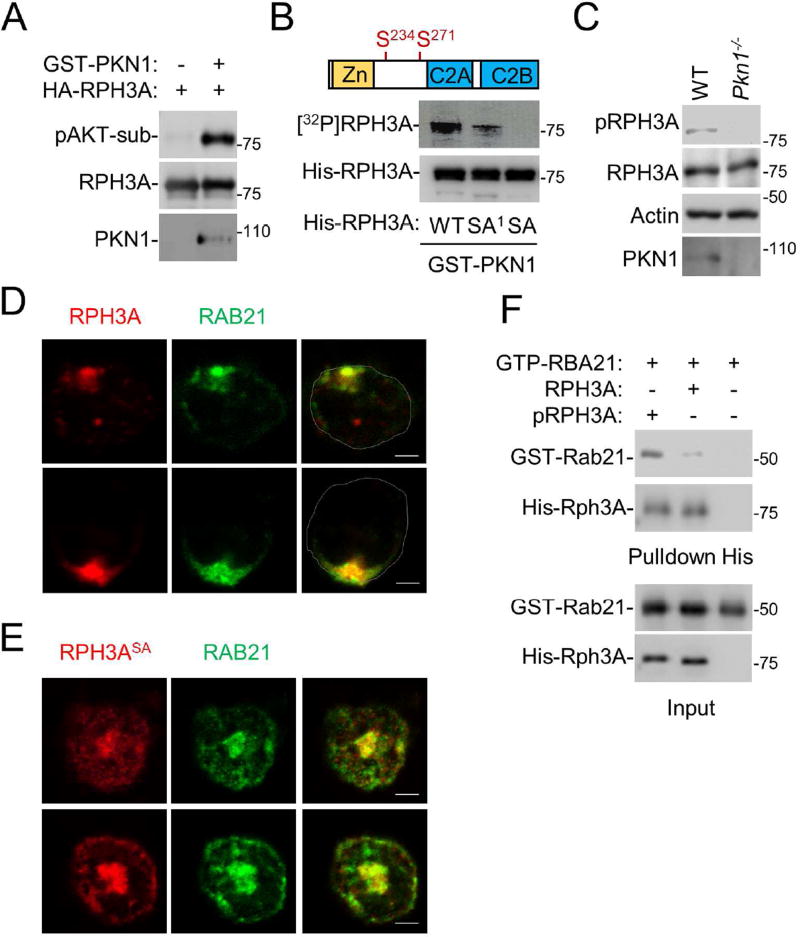
Figure 6. PKN1-mediated phosphorylation of RPH3A is required for polarization.
A. PKN1 phosphorylates RPH3A. Phosphorylation of recombinant RPH3A protein by recombinant PKN1 protein were performed in an in vitro kinase assay, and RPH3A phosphorylation was detected by Western blot analysis using an anti-phospho-AKT substrate motif antibody.
B. RPH3A residues S234 and S271 are phosphorylated by PKN1. WT RPH3A and its mutants SA1 (S234A) and SA (S234, 271A) were subjected to an in vitro kinase assay using [32P]ATP. Phosphorylated RPH3A ([32P]RPH3A) was detected by a phosphoimager, while the inputs were detected by Western blot analysis.
C. RPH3A phosphorylation at Ser 234 depends on PKN1. WT and PKN1-null neutrophils were analyzed by Western blot analysis using an anti-phospho-Ser234 RPH3A, anti-RPH3A, PKN1 or actin antibody.
D,E. WT RPH3A, but not its phosphorylation-defective mutant, can rescue RAB21 polarization in RPH3A-null cells. RPH3A-null neutrophils were transfected with RPH3A-HA (D) or RPH3A-SA-HA (E). The cells were then stained with an anti-HA antibody and anti-RAB21 antibody. Only anti-HA staining positive cells were examined.
F. Phosphorylated RPH3A shows enhanced binding to RAB21. Purified RPH3A protein was first incubated with PKN1 in the presence or absence of ATP in a kinase assay buffer for 30 min at 37 °C. Phosphorylated RPH3A (pRPH3A) is evidenced by its upshift in the blot compared to unphosphorylated RPH3A (RPH3A). These RPH3A proteins were then subjected to a pulldown assay with GTPγS-bound RAB21. The proteins were detected by Western blot analysis.