Fig. 1.
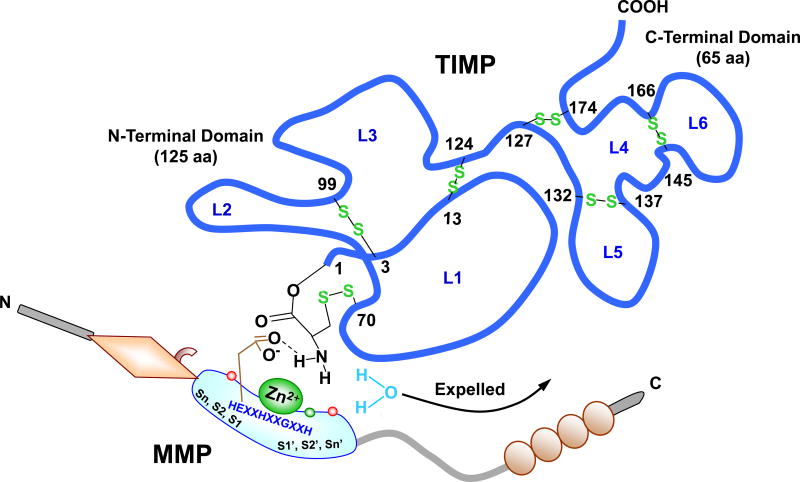
TIMP-MMP Interaction. TIMP is a ~190 aa protein, with an N-terminal domain (loops L1, 2, and 3) and C-terminal domain (loops L4, 5 and 6), which fold independently as a result of 6 disulfide bonds between 12 specific Cys residues. The N-terminal Cys1-Thr-Cys-Val4 and Glu67-Ser-Val-Cys70 are connected via a disulfide bond between Cys1 and Cys70 and are essential for MMP inhibition, as they enter the MMP active site and bidentately chelate the MMP Zn2+. The carbonyl oxygen and α-amino nitrogen in the TIMP Cys1 coordinate with the MMP Zn2+, which is localized in the MMP molecule via the 3 histidines in the HEXXHXXGXXH motif. The TIMP α-amino group then expels Zn2+-bound H2O by binding the MMP H2O binding site and forming a H bond with carboxylate oxygen from conserved MMP Glu202 (E in the HEXXHXXGXXH sequence). TIMP-1 and MMP-3 are used as prototypes. The amino acids involved in Zn2+- and pocket-binding may vary with different MMPs and TIMPs.