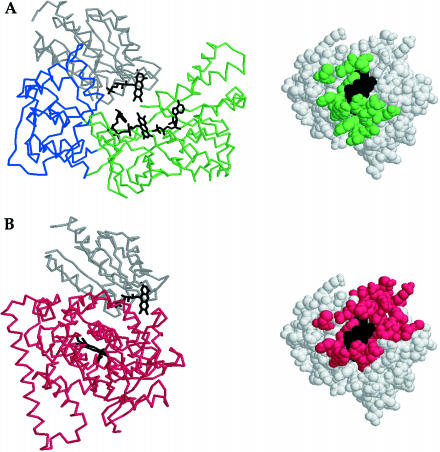
Figure 4.
FMN-reducing and FMN-oxidizing configurations in cytochrome 450 reductase may require a conformational change for interconversion. (A) In cytochrome P450 reductase, electrons are transferred from FAD to FMN in an interdomain reaction. In the structure of cytochrome P450 reductase (10), the FAD and FMN cofactors of the flavodoxin reductase-like (green) and flavodoxin-like (gray) domains are juxtaposed for this electron transfer. Van der Waals contacts between these domains of cytochrome P450 reductase are mapped by homology onto the structure of E. coli flavodoxin, which is shown in the same orientation as in Fig. 3. (B) In cytochrome P450BM-3, electrons are transferred from FMN to the heme in an interdomain reaction. In the structure of the heme/FMN-containing fragment of cytochrome P450BM-3 (34), the FMN-containing domain (gray) is juxtaposed against the heme-containing domain (red) for electron transfer. Van der Waals contacts between these domains of cytochrome P450 BM-3 are mapped by homology onto the structure of E. coli flavodoxin, which is shown in the same orientation as in Fig. 3.