Supplemental Digital Content is available in the text.
Keywords: critical care, inotropic agents, pediatrics, septic shock, vasopressor agents
Objectives:
To assess the validity of Vasoactive-Inotropic Score as a scoring system for cardiovascular support and surrogate outcome in pediatric sepsis.
Design:
Secondary retrospective analysis of a single-center sepsis registry.
Setting:
Freestanding children’s hospital and tertiary referral center.
Patients:
Children greater than 60 days and less than 18 years with sepsis identified in the emergency department between January 2012 and June 2015 treated with at least one vasoactive medication within 48 hours of admission to the PICU.
Interventions:
None.
Measurements and Main Results:
Vasoactive-Inotropic Score was abstracted at 6, 12, 24, and 48 hours post ICU admission. Primary outcomes were ventilator days and ICU length of stay. The secondary outcome was a composite outcome of cardiac arrest/extracorporeal membrane oxygenation/in-hospital mortality. One hundred thirty-eight patients met inclusion criteria. Most common infectious sources were pneumonia (32%) and bacteremia (23%). Thirty-three percent were intubated and mortality was 6%. Of the time points assessed, Vasoactive-Inotropic Score at 48 hours showed the strongest correlation with ICU length of stay (r = 0.53; p < 0.0001) and ventilator days (r = 0.52; p < 0.0001). On multivariable analysis, Vasoactive-Inotropic Score at 48 hours was a strong independent predictor of primary outcomes and intubation. For every unit increase in Vasoactive-Inotropic Score at 48 hours, there was a 13% increase in ICU length of stay (p < 0.001) and 8% increase in ventilator days (p < 0.01). For every unit increase in Vasoactive-Inotropic Score at 12 hours, there was a 14% increase in odds of having the composite outcome (p < 0.01).
Conclusions:
Vasoactive-Inotropic Score in pediatric sepsis patients is independently associated with important clinically relevant outcomes including ICU length of stay, ventilator days, and cardiac arrest/extracorporeal membrane oxygenation/mortality. Vasoactive-Inotropic Score may be a useful surrogate outcome in pediatric sepsis.
Sepsis is a common critical illness leading to pediatric morbidity, mortality, and increased healthcare costs in the United States and worldwide (1–3). Nationally in 2005, there were more than 70,000 hospital admissions for pediatric severe sepsis, accounting for up to 7% of pediatric hospitalizations with an associated cost of nearly $5 billion (1). In recent U.S. database studies, pediatric severe sepsis mortality estimates ranged from 5% to 20% (1, 3). Pediatric sepsis is also associated with significant resource burden, with a median hospitalization cost of $77,000, median PICU length of stay (LOS) of 7 days, and long-term morbidity in survivors (2, 3). Pediatric septic shock is the end stage of severe sepsis, defined as those patients with evidence of cardiovascular dysfunction despite fluid resuscitation (4), and represents the patients at highest risk of mortality (5).
Vasoactive and inotropic medications are standardly used to treat hypotension and cardiovascular dysfunction associated with pediatric septic shock (6). Currently, no uniform, validated measure or scoring system exists to describe the magnitude of hemodynamic support required in pediatric sepsis. In adult sepsis, clinical measures that both objectively describe illness severity and correlate with important outcomes such as mortality are being increasingly recognized as necessary to identify which patients are most at risk for poor outcomes (7). Low-mortality rates in pediatric sepsis limit the feasibility of using death as a primary endpoint, making validated surrogate measures critical for pediatric sepsis research. A validated score that accurately describes cardiovascular dysfunction and correlates with other clinically relevant outcomes such as duration of mechanical ventilation and ICU stay could be used to identify high-risk patients and as an outcome in research and quality improvement.
One candidate scoring system was recently proposed by Gaies et al (8) for use in infant cardiac surgery. The Vasoactive-Inotropic Score (VIS), expanded from the previously described Inotropic Score (9), quantifies the amount of cardiovascular support required by infants postoperatively and includes dopamine, dobutamine, epinephrine, milrinone, vasopressin, and norepinephrine (Fig. 1). VIS has been shown to correlate with worse short-term clinical outcomes in infants following cardiac surgery (8, 10–12). Various studies have used VIS in pediatric sepsis to describe inotropic and vasopressor support in their population (13, 14). However, to our knowledge, only one previous study by Haque et al (15) has shown an association between VIS and mortality in pediatric sepsis. This study looked at a small cohort in India with a much higher mortality rate (42%) for pediatric sepsis than previously described in U.S. populations (1–3). This finding has not been replicated in a lower mortality, resource-rich cohort nor has it been demonstrated to correlate with other important outcomes such as length of ventilation or ICU stay. Additionally, optimal timing for assessment of VIS in pediatric sepsis has not been established.
Figure 1.

Calculation of Vasoactive-Inotropic Score (VIS).
This study aims to determine whether VIS is a valid surrogate outcome measure in pediatric sepsis by testing its association with important short-term outcomes. We also seek to determine the optimal timing for assessment of VIS. We hypothesize that VIS at early time points in sepsis (6, 12, 24, and 48 hr following arrival to the ICU) will be associated with key outcome measures including ICU LOS, ventilator days, and a composite outcome of cardiac arrest, need for extracorporeal membrane oxygenation (ECMO), and in-hospital mortality. VIS is easily measured and, if validated in pediatric sepsis, has the potential to standardize the quantification of hemodynamic support, identify, and stratify high-risk patient populations, and be used as a surrogate outcome measure for quality initiatives and research.
MATERIALS AND METHODS
We conducted a secondary analysis of a single-center sepsis registry that includes children clinically identified with suspected sepsis in the emergency department (ED) from January 2012 to June 2015. This study was conducted at Children’s Hospital Colorado, a freestanding tertiary children’s hospital. Institutional ED protocols for sepsis include an “activation” process for suspected sepsis, which includes protocolized mobilization of additional personnel and equipment, paging, order sets and standardized procedures for rapid initiation of IV access, fluid resuscitation, antibiotics, and critical therapeutics. Bedside clinicians (nurses, advanced practice providers, or physicians) initiate sepsis activations when they clinically suspect infection in the presence of altered mental status or perfusion. Using these clinical indications for sepsis treatment follows international guidelines for recognition and treatment of pediatric septic shock (6). The sepsis registry was created to support sepsis clinical quality improvement work and approved by the Children’s Hospital Colorado Organizational Research Risk and Quality Improvement Review Panel. Extraction of data from the prospective registry into a limited dataset for analysis for generalizable research was approved by the Colorado Multiple Institution Review Board.
ED patients were identified for inclusion in the sepsis registry through the presence of any of the following in the electronic health record (EHR): 1) use of a sepsis-specific order set at the time of ED treatment, 2) use of the sepsis activation paging system which is time-stamped in the EHR, and 3) missed cases. Missed cases were identified in the following manner: all patients placed in intensive care within 24 hours of ED care who received antibiotics or were hypotensive in the ED are identified through a monthly EHR query. Two trained reviewers reviewed these cases monthly, and cases considered missed sepsis were added to the database along with their clinical data. Missed case classification by the two trained reviewers was tested on an ongoing basis and a κ of greater than 0.8 was sustained.
Data were extracted from the EHR using extract, transfer, and load methodology. From the Epic Clarity database, reports generated using Crystal Reports 2008 were imported monthly into a Research Electronic Data Capture (REDCap) database (16). Additional monthly loads updated time-dependent fields that were incomplete at the original load, such as hospital LOS and 30-day mortality. Routine quality auditing identified specific variables as unreliably captured in the EHR; these underwent chart review and manual data entry monthly, including the less than 1% of encounters that included article (non-EHR) resuscitation charting.
Additional exclusion criteria were applied to the larger registry to obtain the cohort for this study. Potential subjects were identified for inclusion in the cohort by identifying patients in the registry who had a vasoactive agent administered during hospitalization as indicated in the medical administration record. These cases subsequently underwent chart review to determine whether inclusion/exclusion criteria were met. Inclusion criteria were patients who received at least one vasoactive or inotropic agent in the first 48 hours after arrival to the ED or ICU. Exclusion criteria were admission to the neonatal ICU, age less than 60 days or greater than 18 years, need for an operating room (OR) procedure within the first 6 hours after admission, determination that the primary reason for requiring inotropic or vasoactive support was a definitive alternative diagnosis (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A449), placement onto ECMO support prior to initiation of vasoactive/inotropic support, and initiation of vasoactive/inotropic support occurring only following an OR procedure.
Demographic and clinical information collected on all patients from the auto-extracted database included age at admission, Pediatric Index of Mortality 3 score (17), gender, death during hospitalization, vasopressor days, ICU and hospital LOS, presence of complex chronic condition as defined by Feudtner et al (18), initial lactate in 8 hours, amount of fluid boluses received in the first 24 hours, and time to antibiotic from initial vitals in the ED. Information that was manually chart reviewed included cardiac arrest, need for ECMO, underlying diagnosis, infectious source, need for and timing of OR procedures, need for intubation, and length of ventilation.
VIS was the independent variable in this study. Maximal VIS in the first 48 hours and VIS at 6, 12, 24, and 48 hours following ED admission time and ICU admission time were recorded. VIS was manually calculated through investigator chart review on patients per Gaies et al (8) (Fig. 1). For a sample patient in the cohort on 9 µg/kg/min of dopamine, 0.12 µg/kg/min of norepinephrine, and 0.05 µg/kg/min of epinephrine at 48 hours after time of admission, VIS at 48 hours would be calculated as follows: 9 + (100 × 0.12) + (100 × 0.05) = 23.
Patients who were in the OR in the first 48 hours but after 6 hours following admission were included with the VIS calculated only for those time points preceding the OR procedure in order to exclude patients who required intraoperative and postoperative vasoactive and inotropic support due to shock caused by conditions other than sepsis such as hemorrhage, anesthetic agents, or nonseptic systemic inflammatory response. For patients who died or were cannulated onto ECMO prior to the 48-hour time mark, VIS was calculated only for time points preceding death or ECMO.
Primary outcomes were ICU LOS and ventilator-free days. The secondary outcome was a combined poor outcome variable defined as the following: cardiac arrest or need for cardiopulmonary resuscitation, need for ECMO, or death during hospitalization. For patients who had a cardiac arrest in the first 48 hours, subsequent VIS following the arrest event was excluded from analysis for the composite outcome but included in the analysis for ICU LOS, ventilator days, and need for intubation.
Ventilator days were chart abstracted manually, and a ventilator day was defined as any amount of invasive mechanical ventilation on a given calendar day. Patients who died during hospitalization were assigned a maximum ventilator days value of 28 days. ICU LOS was auto-extracted. Patients who died were assigned the value of the maximum ICU LOS in the cohort, which was 964 hours or 48 days. Patients with tracheostomies who were baseline ventilated were defined as requiring intubation if they required escalation above their home settings, and total time ventilated was calculated as time from escalation to time of return to home ventilation settings. Patients who were intubated solely for an operative procedure were not counted as requiring intubation.
Study data were collected and managed using REDCap, hosted at the University of Colorado. REDCap is a secure, web-based application designed to support data capture for research studies (16). A limited HIPAA-compliant dataset was exported from REDCap into SAS v. 9.4 (SAS Institute, Cary, NC) for analysis.
Patients’ demographics and baseline clinic characteristics were summarized using descriptive statistics. Median and interquartile range were reported for continuous variables, whereas frequency and percentage were reported for binary and categorical variables. The data distribution of continuous outcomes such as ICU LOS and ventilator days was examined to determine the models to be employed. As a result, ICU LOS was transformed in natural log scale and general linear model was fitted for this dependent variable. There were over 50% of subjects with a ventilator days value of zero, thus zero inflated Poisson model was fit for this dependent variable. Candidate covariates such as PIM3 score, complex chronic condition, initial lactate, fluid boluses within first 24 hours, and time to antibiotics and pneumonia were chosen based on clinical relevance and published sepsis literature (19–21). Spearman rank correlation test was performed to further narrow down the candidate covariates that were included in the multivariable models. Covariates included in the final model are shown in Supplemental Tables 2a–2e (Supplemental Digital Content 2, http://links.lww.com/PCC/A450). Multiple logistic regression models were fit for the binary outcomes of intubation and composite outcome. Best fit models were evaluated based on the statistical significance (p) and appropriate model fitting statistics (Akaike information criterion, R2 and Tjur’s R2). p value of less than 0.05 was considered statistically significant, and all analyses were performed using SAS V9.4.
RESULTS
Between January 2012 and June 2015, 215 patients were identified in the sepsis database as having received any vasoactive or inotropic support, of whom 138 patients met inclusion criteria and did not meet exclusion criteria (Fig. 2). Seven included patients had VIS calculations at limited time points, due to OR procedure or death in first 48 hours of ICU admission. For the composite outcome analysis only, two additional patients were excluded due to cardiac arrest occurring in the first 6 hours after admission, and one patient had VIS calculated at only the 6- and 12-hour time points due to cardiac arrest occurring between 12 and 24 hours.
Figure 2.
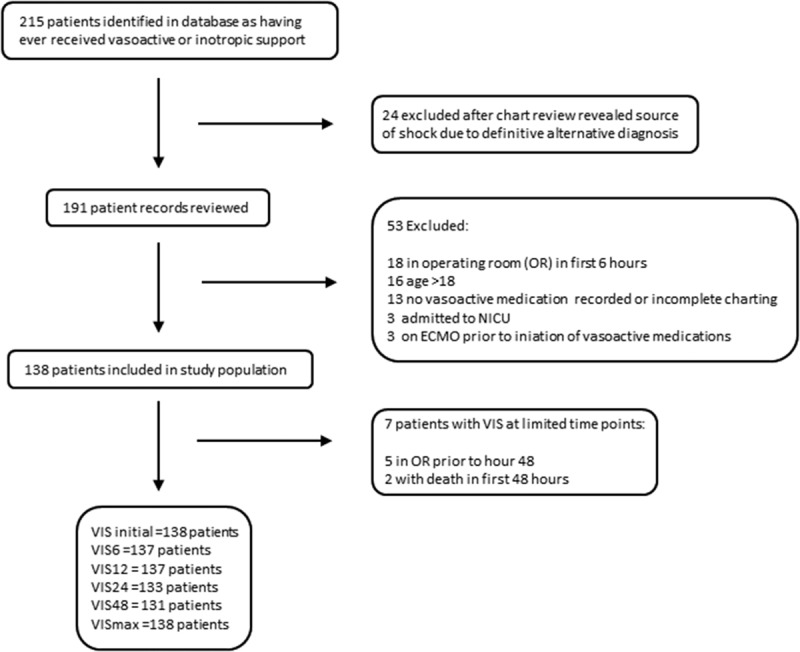
Exclusion diagram. NICU = neonatal ICU, OR = operating room, VIS = Vasoactive-Inotropic Score.
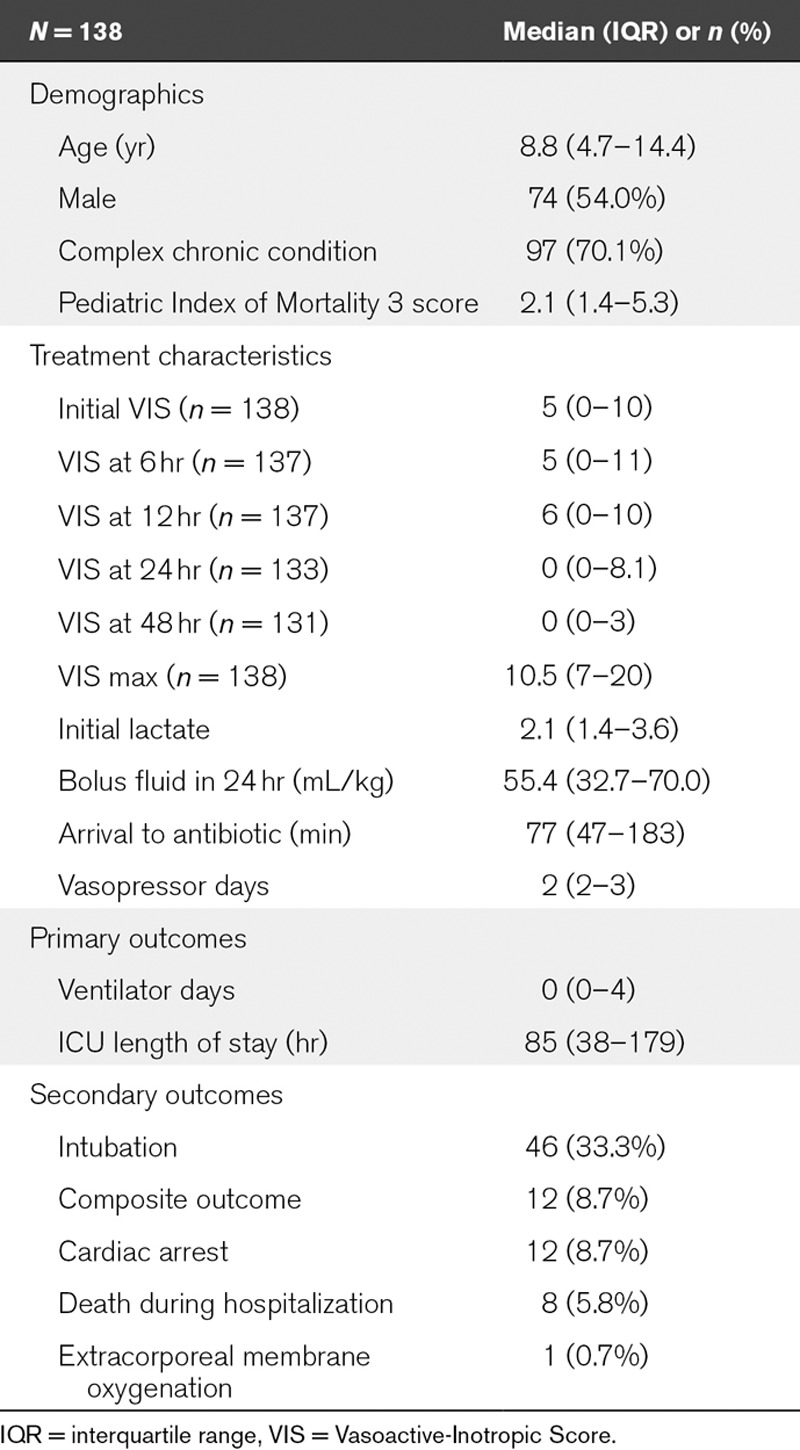
Demographic and treatment characteristics are shown in Table 1. The most common underlying conditions were genetic/metabolic (21.6%) and oncologic (13.0%). The most common infectious sources were pneumonia (31.7%) and bacteremia (23.7%). Ninety-six percent of intubations occurred in the first 48 hours, and the absolute range of VIS at 48 hours was 0–40. Details of specific vasoactive and inotropic agent usage in study patients as well as the timing of intubations are described in Supplemental Table 3 (Supplemental Digital Content 3, http://links.lww.com/PCC/A451) and Supplemental Table 4 (Supplemental Digital Content 4, http://links.lww.com/PCC/A452), respectively. Change in VIS over time is demonstrated graphically in Supplemental Graph 1 (Supplemental Digital Content 5, http://links.lww.com/PCC/A453).
TABLE 1.
Demographic, Treatment, and Outcome Characteristics of Patient Population
As a first step, VIS correlations with the primary outcomes of ICU LOS and ventilator days were calculated at prespecified time points after ED and ICU arrival on univariate analysis to identify the best VIS time point to use on multivariable analysis. VIS at time points calculated based on ICU arrival time (Table 2) performed best, therefore, arrival at ICU was used as time point zero for additional analyses (VIS for ED time points not shown). We then compared VIS at different time points after ICU admission to each other (Table 2).
TABLE 2.
Correlation of Vasoactive-Inotropic Score Calculated After ICU Arrival With ICU Length of Stay and Ventilator Days
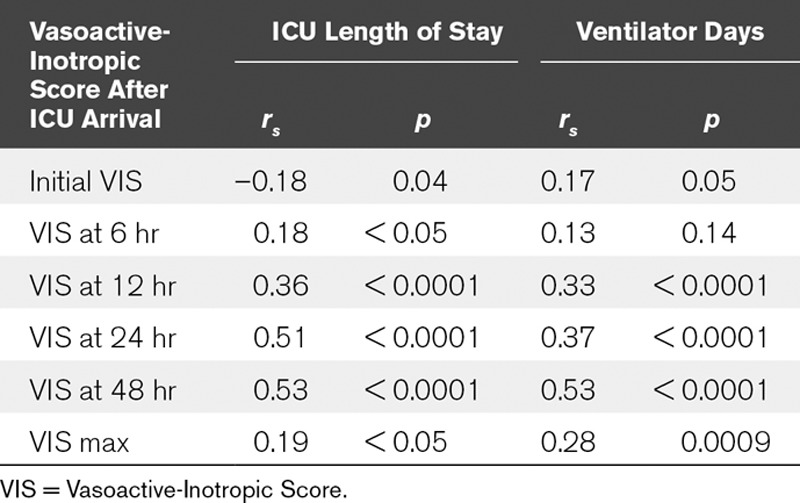
Given that VIS at 48 hours had the strongest correlation with the primary outcomes (ICU LOS and ventilator days), VIS at 48 hours was chosen as the independent continuous variable in the multivariable analysis. VIS at 48 hours was independently associated with both ventilator days and ICU LOS, showing that for every unit increase in VIS, there was an 8% increase (p < 0.01) and 13% increase (p < 0.0001) in ventilator days and ICU LOS, respectively. We also tested the relationship of patients who required any vasoactive or inotropic support at 48 hours with both primary outcomes via multivariable analysis. VIS at 48 hours as a dichotomous variable did not have a statistically significant association with ventilator days on multivariable analysis. VIS at 48 hours as a dichotomous variable was associated with ICU LOS but had minimal effect on overall fit of the multivariable model compared with VIS at 48 hours as a continuous variable (change in R2 of 0.04).
For the composite outcome, the model indicated a nonstatistically significant association with VIS at 48 hours, with an 8% increase in the odds have having the composite outcome with every unit increase in VIS (p = 0.24). This analysis was likely underpowered due to limited events; there were only 12 total composite outcome events in the cohort and five of those events happened prior to 48 hours and so were excluded from the analysis for VIS at 48 hours. Of the five excluded subjects from the VIS at 48-hour analysis, three patients suffered cardiac arrest or death between 12 and 24 hours (Supplemental Table 5, Supplemental Digital Content 6, http://links.lww.com/PCC/A454). We therefore performed a secondary analysis using VIS at 6 and 12 hours in the multivariable model in order to capture those events that occurred earlier in the ICU stay. We found that VIS at 12 hours had a strong independent association with cardiac arrest, ECMO, or death. For every unit increase in VIS at 12 hours, there was a 14% increase in the odds of subsequently experiencing the composite outcome (p < 0.01). Interestingly, in this model, for a fixed VIS at 12 hours, higher VIS at 6 hours was associated with a decreased risk of later having the composite outcome (OR = 0.87; 95% CI, 0.77–0.99). Additionally, when looking at the prearrest VIS trend in the five patients who experienced the composite outcome during the first 48 hours, four of the five demonstrated increasing VIS in the 8 hours prior to the event (Supplemental Table 5, Supplemental Digital Content 6, http://links.lww.com/PCC/A454; and Supplemental Graph 2, Supplemental Digital Content 7, http://links.lww.com/PCC/A455).
DISCUSSION
In pediatric sepsis, there is a need for validated surrogate outcome measures (7). Our study provides a critical first step in validating VIS for such purposes. Although VIS has been validated in pediatric cardiac surgery (8, 10–12), and has been used in previous studies of pediatric patients with severe sepsis to describe the severity of illness and as a measure of hemodynamic support (13, 14), only one prior study by Haque et al (15) has shown an association between maximal VIS and outcomes in pediatric sepsis. Our study complements the study by Haque et al (15) done in a resource-poor setting, by showing that in a resource-rich setting with a medically complex, low-mortality cohort of children with septic shock, VIS at 48 hours after ICU arrival is independently associated with short-term outcomes including ICU LOS and ventilator days.
Importantly, the association of VIS at 48 hours with these outcomes was independent of the validated PIM3 score. This finding is in part not surprising, as the PIM3 score was validated to predict mortality across the broad spectrum of PICU admissions and did not specifically target sepsis patients or outcomes other than mortality (17). However, it is also important to note that progressive cardiovascular instability plays a role in the pathophysiology of sepsis and the cardiovascular component of the PIM3 score is limited to systolic blood pressure and acid base status in the first hour. Our results indicate that VIS at 48 hours may capture a component of illness in pediatric sepsis that is not fully addressed by PIM3. VIS at 48 hours may therefore complement existing acuity scores as both a relatively early indicator of the likely duration of critical care support as well as a surrogate outcome that highlights cardiovascular instability.
Consistent with this concept, our study shows that persistent rather than early or maximal need for vasoactive and inotropic support in the first 48 hours is most strongly associated with duration of critical care support. Patients with a high VIS at 48 hours demonstrate ongoing cardiovascular dysfunction and are inherently at highest risk of a poor outcome. In contrast to previous studies of VIS in infant cardiac surgery (8, 11, 12) and the study by Haque et al (15) of VIS in pediatric sepsis (15), we did not find a strong correlation between maximal VIS and outcomes of interest. Early aggressive resuscitation is associated with improved outcomes and shock reversal in pediatric sepsis (6), and it is possible that early and maximal VIS may reflect attentive support of reversible pathophysiology compared with VIS at later time points.
The relationship between high VIS and prolonged intubation and ICU LOS is intuitive. Patients requiring vasoactive or inotropic support due to vasoplegia or capillary leak syndrome are also those who are more likely to have acute respiratory distress syndrome or pulmonary edema requiring positive-pressure ventilation. Additionally, patients with myocardial dysfunction that require inotropic support are often intubated to reduce total body oxygen demand and improve left ventricular function. Conversely, prolonged ventilation can also directly lead to increased vasoactive and inotropic medication requirements due to increased intrathoracic pressure, decreased venous return, and increased right ventricular afterload. As the vast majority of patients requiring invasive ventilation were intubated prior to 48 hours (Supplemental Table 4, Supplemental Digital Content 5, http://links.lww.com/PCC/A452), VIS at 48 hours cannot be considered to be predictive of intubation. However, those patients with a persistent need for vasoactive or inotropic support 48 hours after admission may have prolonged multiple organ dysfunction increasing the duration and need for ICU care and ventilation. The association of VIS with ICU LOS and length of ventilation suggests that cardiovascular dysfunction, as represented by a high VIS, may be causal in both of these important outcomes. This may mean that interventions targeting a decrease in a patient’s VIS may ultimately affect these relevant outcomes as well.
VIS at 48 hours after PICU arrival did not show a statistically significant association with the secondary composite outcome but low frequency of this outcome means that this analysis was likely underpowered. More important, many of these events occurred early in hospitalization and VIS at 48 hours is by definition not useful to accurately identify or predict early cardiovascular collapse. We therefore explored an earlier time point (VIS at 12 hr) in a secondary analysis and found that it was strongly and independently associated with subsequent cardiac arrest, ECMO, or death. Again, this finding was independent of the measured acuity score (PIM3) suggesting a complementary role for the quantification of cardiovascular support in this population. Of interest, for a fixed VIS at 12 hours, VIS at 6 hours was inversely associated with the composite outcome. Although it is possible that an early high VIS at 6 hours portends a better outcome for patients as it represents aggressive early resuscitation, this is unlikely as higher VIS at 6 hours is associated with worse outcomes when modeled separately from VIS at 12 hours. An alternative explanation for this relationship may be that direction or trend of VIS is important. Those patients who do not wean from support quickly or require increasing support between 6 and 12 hours are patients whose illness is worsening and are more likely to have a poor outcome. This concept is supported by our descriptive data showing that in four of five patients with early cardiovascular collapse, VIS was rising in the 4 hours immediately prior to the event, although the small number of cases precludes meaningful statistical analysis. Overall, although these data are suggestive that early and rising VIS may predict subsequent poor outcome, due to the small number of events, it can be considered exploratory only and will require validation in larger cohorts.
Overall, our study population is similar to pediatric severe sepsis populations presenting to the ED of tertiary centers with pediatric ICUs. The 6% mortality in our study of patients with septic shock is similar to that reported from ED-based pediatric sepsis registries with sepsis quality programs in place (19, 22, 23) but is lower than what has been reported in national database and ICU-based pediatric studies (1–3). Also, the median PIM3 score in our population was lower than in other ICU-based studies (2), suggesting that our population may have had a lower overall level of illness severity. Compared with previously published pediatric ED sepsis studies in the United States, our population had a similar proportion of patients with complex chronic conditions (3, 22, 23). A potentially unique aspect of our population was the median amount of bolus fluid (55 mL/kg) received by patients in the first 24 hours, which is lower than American College of Critical Care Medicine guidelines but is similar to what has been described in other pediatric tertiary hospital populations (23, 24). Despite this possible variation, VIS was still found to be independently associated with outcomes of interest when controlling for the amount of fluid resuscitation. Additionally, although vasoactive and inotropic usage may vary in other institutions in comparison to our ICU due to individual practice patterns and availability of specific drugs, such as those in resource-poor settings, VIS is calculated in a manner that incorporates all commonly used vasoactive and inotropic equally and thus can be used to compare across institutions despite different prescribing patterns.
In summary, this study shows that VIS is a reliable marker of cardiovascular support that is independently associated with important outcomes in pediatric sepsis and may complement existing acuity scores as an early prognostic indicator of the duration of critical care support in this population. Low-mortality rates in pediatric sepsis mean that using death as a primary endpoint for many clinical investigations is often impractical. Our results indicate that VIS at 48 hours could serve as a surrogate cardiovascular outcome to help develop investigations with sufficient power to answer clinical questions in a timely and feasible manner. It can also be used to quantify hemodynamic support, allowing comparison of patient populations across studies and centers. Additionally, VIS is a simple score that can be easily calculated in real time at the bedside and requires only 48 hours of data collection. Finally, although only preliminary in nature, our study also suggests that trends in VIS prior to the 48-hour time point should be explored further in larger multiinstitutional studies as a potential early warning or risk-stratification method to clinically identify septic patients at increasing risk for sudden cardiovascular collapse.
There are limitations to this study. Data were collected retrospectively from an EHR and quality improvement database primarily designed to assess early recognition and treatment of sepsis in the ED. It was also a single-center study of a tertiary children’s hospital center and may not be generalizable to all institutions that care for pediatric sepsis patients. Given that sepsis patients are very heterogeneous, determining time point zero of illness in sepsis is difficult, and it is possible that VIS values measured at discrete time points after ICU arrival in this study were affected by variations in the timing of a patient’s presentation to the ED and subsequent ICU admission. Although we excluded those patients diagnosed with dilated cardiomyopathy/myocarditis, all patients did not have an objective measure of cardiac function so we were unable to make comparisons or draw conclusions between groups of patients with and without myocardial dysfunction, nor did we have long-term follow-up on which patients in the cohort had persistent ventricular abnormalities. Additionally, these findings may not be generalizable to individual patients requiring early operative intervention or who suffer very early catastrophic events such as cardiac arrest, death, or cannulation onto ECMO, as these patients were excluded from this study. As is true in all observational studies, treatments were not dictated by the study, so individual clinician and institutional practices such as threshold for initiation of cardiovascular support may have affected the VIS. Additionally, when evaluating the VIS as a score itself, it is important to note that individual components of the score are weighted for convenience rather than considering the clinical relevance of each medication. Due to the lack of long-term follow-up in this registry-based study, we were unable to assess the association of VIS with longer term mortality after hospitalization or functional outcomes. Investigation of the relationship between VIS and functional outcomes is an important area for future prospective studies.
CONCLUSIONS
VIS at 48 hours after ICU admission in pediatric sepsis is an easily calculated clinical score that is independently associated with ICU LOS and length of ventilation. VIS at 12 hours is independently associated with risk of cardiac arrest, death, or need for ECMO. In pediatric sepsis, VIS is a reliable marker of cardiovascular support that may be used as a surrogate outcome for research studies and provide additive value to existing pediatric acuity scores in this population.
ACKNOWLEDGMENTS
We are grateful for the contributions of Kendra Kocher, Kathleen Grice, and Mimi Goodwin in the Section of Pediatric Emergency Medicine for their tireless commitment to the maintenance of the sepsis registry, managing data entry, quality control, and compliance.
Supplementary Material
Footnotes
*See also p. 803.
This study was performed at Children’s Hospital Colorado, Aurora, CO.
Drs. Davidson and Scott contributed equally as senior authors and mentors for the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Financial support for this study was provided by National Institutes of Health/Heart Lung and Blood Institute 1K23HL123634 (to Dr. Davidson) and the Brigid Hope Fund. Financial support for the database used in this study was provided by the Clinical and Operational Effectiveness and Patient Safety Grant of the University of Colorado (Grant Number 2300704).
Dr. Davidson received support for article research from the National Institutes of Health (NIH), and his institution received funding from NIH/National Heart, Lung, and Blood Institute K23. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013; 14:686–693 [DOI] [PubMed] [Google Scholar]
- 2.Weiss SL, Fitzgerald JC, Maffei FA, et al. SPROUT Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators Network: Discordant identification of pediatric severe sepsis by research and clinical definitions in the SPROUT international point prevalence study. Crit Care 2015; 19:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014; 15:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein B, Giroir B, Randolph AMembers of the International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 5.Carcillo JA, Kuch BA, Han YY, et al. Mortality and functional morbidity after use of PALS/APLS by community physicians. Pediatrics 2009; 124:500–508 [DOI] [PubMed] [Google Scholar]
- 6.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009; 37:666–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-Inotropic Score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010; 11:234–238 [DOI] [PubMed] [Google Scholar]
- 9.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 1995; 92:2226–2235 [DOI] [PubMed] [Google Scholar]
- 10.Davidson J, Tong S, Hancock H, et al. Prospective validation of the Vasoactive-Inotropic Score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med 2012; 38:1184–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butts RJ, Scheurer MA, Atz AM, et al. Comparison of maximum Vasoactive Inotropic Score and low cardiac output syndrome as markers of early postoperative outcomes after neonatal cardiac surgery. Pediatr Cardiol 2012; 33:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaies MG, Jeffries HE, Niebler RA, et al. Vasoactive-Inotropic Score is associated with outcome after infant cardiac surgery: An analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med 2014; 15:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulla KM, Sachdev A, Gupta D, et al. Continuous renal replacement therapy in children with severe sepsis and multiorgan dysfunction—A pilot study on timing of initiation. Indian J Crit Care Med 2015; 19:613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai Y, Cornell TT, Cooley EG, et al. Therapeutic plasma exchange may improve hemodynamics and organ failure among children with sepsis-induced multiple organ dysfunction syndrome receiving extracorporeal life support. Pediatr Crit Care Med 2015; 16:366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haque A, Siddiqui NR, Munir O, et al. Association between Vasoactive-Inotropic Score and mortality in pediatric septic shock. Indian Pediatr 2015; 52:311–313 [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straney L, Clements A, Parslow RC, et al. ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network: Paediatric index of mortality 3: An updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med 2013; 14:673–681 [DOI] [PubMed] [Google Scholar]
- 18.Feudtner C, Christakis DA, Connell FA.Pediatric deaths attributable to complex chronic conditions: A population-based study of Washington State, 1980-1997. Pediatrics 2000; 106:205–209 [PubMed] [Google Scholar]
- 19.Paul R, Melendez E, Stack A, et al. Improving adherence to PALS septic shock guidelines. Pediatrics 2014; 133:e1358–e1366 [DOI] [PubMed] [Google Scholar]
- 20.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014; 42:2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott HF, Donoghue AJ, Gaieski DF, et al. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad Emerg Med 2012; 19:1276–1280 [DOI] [PubMed] [Google Scholar]
- 22.Cruz AT, Perry AM, Williams EA, et al. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics 2011; 122:758–766. [DOI] [PubMed] [Google Scholar]
- 23.Larsen GY, Mecham N, Greenberg R.An emergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics 2011; 127:e1585–e1592 [DOI] [PubMed] [Google Scholar]
- 24.Akcan Arikan A, Williams EA, Graf JM, et al. Resuscitation bundle in pediatric shock decreases acute kidney injury and improves outcomes. J Pediatr 2015; 167:1301–1305.e1 [DOI] [PubMed] [Google Scholar]


