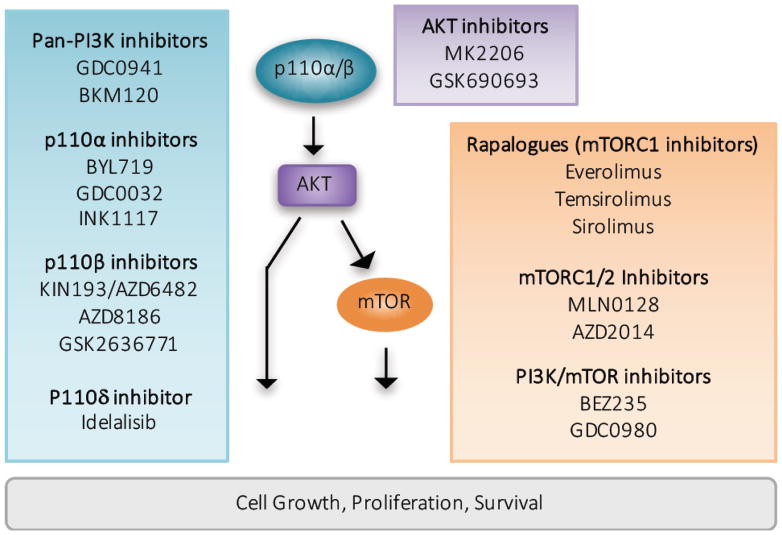
Figure 2. Therapeutic inhibition of the PI3K/AKT pathway in cancer.
PI3K/AKT pathway hyperactivation represents a viable therapeutic modality in cancer. The blue box indicates PI3K inhibitors with preclinical and/or clinical activity. Non-selective, “pan” PI3K inhibitors target all class I PI3K isoforms and p110α-selective inhibitors are biologically active against tumors with p110α hyperactivation through upstream RTK activation or direct p110α mutational activation. P110β-selective inhibitors are efficacious in the treatment of tumors characterized by PTEN genomic alteration, and the p110δ-selective inhibitor Idelalisib is effective in blocking PI3K-mediated B cell receptor signaling and is FDA approved for the treatment of relapsed chronic lymphoid leukemia (CLL). The purple box highlights AKT inhibitors, which are most effective in the treatment of tumors characterized by AKT genomic alteration but may also possess therapeutic benefit in PI3K hyperactivated tumors. mTORC1, dual mTORC1/2, and dual-specificity mTOR/PI3K inhibitors are represented in the orange box. Currently several rapalogues are FDA-approved for multiple cancer indications.