Figure 6.
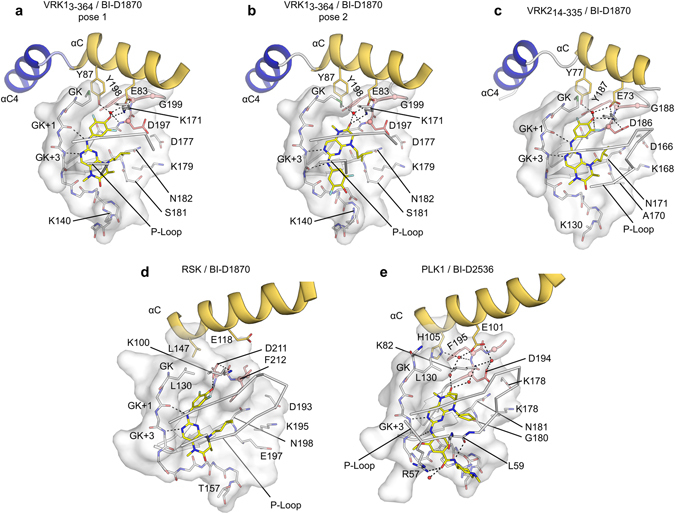
Active VRKs, PLK1 and RSK2 have dissimilar binding modes to pteridinone compounds. (a,b) BI-D1870 has two distinct binding modes to VRK1, supported by different interactions with the protein hinge region. (c) BI-D1870 interaction with VRK2. Both VRK1 and VRK2 are constitutively in a DFG-in, active conformation. (d) RSK2 binds to BI-D1870 in an inactive, DFG-out conformation (PDB:5DK9). (e) BI-D2536 binding mode to PLK1 (PDB:2RKU) is similar to pose 2 in VRK13-364/BI-D1870 co-crystals. PLK1 residues at the back of the ATP-binding site (K82, E101 and D194) contact the compound via an extensive network of water-mediated hydrogen bonds. Side chains of Asp and Tyr/Phe residues from the DFG (DYG in the VRKs) are shown in stick representations; Cαs in these residues are shown as spheres (salmon).
