Figure 2.
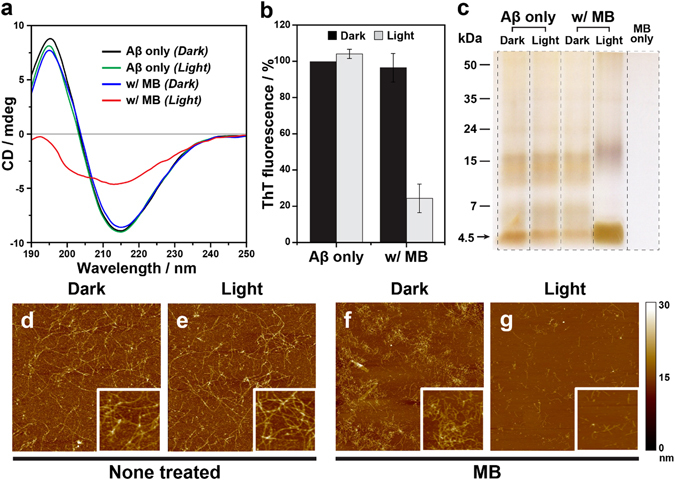
Light-induced suppression of Aβ42 self-assembly by MB. (a) CD spectra of Aβ42 aggregates incubated under various conditions. Two characteristic peaks in the CD spectrum presenting the β-sheet structure disappeared in the MB (10 μM)-treated Aβ42 (40 μM) under light illumination. (b) ThT fluorescence assay to measure the formation of amyloid fibrils. Significant decrease in ThT fluorescence indicates that the aggregation of Aβ42 monomers was substantially suppressed. (c) Silver-stained native gel electrophoresis showing that the monomeric contents was highly increased in MB-treated Aβ42 under light illumination. The arrow indicates a 4.5 kDa molecular mass that corresponds to the monomers of Aβ42. (d–g) Representative AFM images of Aβ42 incubated with or without MB under dark and light conditions. Only fragmented fibrils were observed in MB-treated Aβ42 (see insets enlarged from panels).
